Carbonyl Compounds Produced by Vaporizing Cannabis Oil Thinning Agents
Date: March 29, 2017
Source: The Journal of Alternative and Complementary Medicine.
To cite article:
Troutt William D. and DiDonato Matthew D.. The Journal of Alternative and Complementary Medicine. March 2017, ahead of print. https://doi.org/10.1089/acm.2016.0337
Author information:
William D. Troutt, NMD, and Matthew D. DiDonato, PhD
Medical Marijuana Research Institute, Tempe, AZ.
Address correspondence to:
Matthew D. DiDonato, PhD
Medical Marijuana Research Institute
627 S. 48th Street Suite 100
Tempe, AZ 85281
E-mail: matthew.didonato@gmail.com
ABSTRACT
Objective: Cannabis use has increased in the United States, particularly the use of vaporized cannabis oil, which is often mixed with thinning agents for use in vaporizing devices. E-cigarette research shows that heated thinning agents produce potentially harmful carbonyls; however, similar studies have not been conducted (1) with agents that are commonly used in the cannabis industry and (2) at temperatures that are appropriate for cannabis oil vaporization. The goal of this study was to determine whether thinning agents used in the cannabis industry produce potentially harmful carbonyls when heated to a temperature that is appropriate for cannabis oil vaporization.
Design: Four thinning agents (propylene glycol [PG], vegetable glycerin [VG], polyethylene glycol 400 [PEG 400], and medium chain triglycerides [MCT]) were heated to 230°C and the resulting vapors were tested for acetaldehyde, acrolein, and formaldehyde. Each agent was tested three times.
Setting/Location: Testing was conducted in a smoking laboratory.
Outcome measures: Carbonyl levels were measured in micrograms per puff block.
Results: Analyses showed that PEG 400 produced significantly higher levels of acetaldehyde and formaldehyde than PG, MCT, and VG. Formaldehyde production was also significantly greater in PG compared with MCT and VG. Acrolein production did not differ significantly across the agents.
Conclusions: PG and PEG 400 produced high levels of acetaldehyde and formaldehyde when heated to 230°C. Formaldehyde production from PEG 400 isolate was particularly high, with one inhalation accounting for 1.12% of the daily exposure limit, nearly the same exposure as smoking one cigarette. Because PG and PEG 400 are often mixed with cannabis oil, individuals who vaporize cannabis oil products may risk exposure to harmful formaldehyde levels. Although more research is needed, consumers and policy makers should consider these potential health effects before use and when drafting cannabis-related legislation.
INTRODUCTION
In the twenty years since California became the first state to legalize medical cannabis, an additional 28 states and the District of Columbia have passed laws permitting cannabis use for medicinal purposes, and eight states have legalized adult use. Consequently, cannabis use in the United States has increased significantly. A study sponsored by the National Institute on Alcohol Abuse and Alcoholism estimated that 9.5% of American adults used cannabis in 2013, up from 4.1% in 2002,1 and a recent Gallup poll found that 13% of adults in the United States currently use cannabis.2Over time, it is likely that more adults will use cannabis, as national polls show that 84% of Americans believe that cannabis should be legalized medicinally3 and 58% support national adult use legalization.4
Given this rapid increase in cannabis use, it is important to examine potential medical and health-related issues. Studies show that as much as 86% of medical cannabis consumers rate smoking as the preferred method of cannabis use.5,6 Therefore, one issue concerns the effect of inhaled cannabis on respiratory function and health. Some research shows that cannabis smoke contains carcinogenic compounds that are similar to those of tobacco smoke, with some compounds in greater quantities than those produced by tobacco,7,8 although studies examining the links between cannabis use and lung cancer are inconclusive.9–15 Some frequent cannabis smokers also experience respiratory issues such as coughing, wheezing, increased sputum production, dyspnea, pharyngitis, and exacerbation of asthma.11,16–19
Due to these issues, cannabis vaporization is becoming more widespread as a potentially safer alternative to smoking. Vaporization is the process of heating cannabis to a temperature at which the plant's chemical compounds boil, creating an aerosol that can be inhaled. Because the cannabis is not heated to the point of combustion, fewer carcinogens and irritants are produced. Compared with smoking, vaporization is associated with fewer respiratory issues in cannabis users,17 which some researchers suggest is a result of lower exposure to toxic substances.17,20,21In addition, Abrams et al.22 found that the amount of inhaled carbon monoxide was significantly lower for vaporized cannabis compared with cannabis that was smoked.
Although several cannabis-derived products can be vaporized, cannabis oil is quickly increasing in popularity. In Colorado, for example, the sale of prefilled cannabis oil cartridges (a product that is exclusively vaporized) increased by 163% from February 2015 to February 2016,23 and in Washington State sales doubled from June 2015 to September 2015.24 For the oil to be vaporized and inhaled, cannabis oil cartridges are typically connected to a vaporizing device that contains a heating element and a disposable or rechargeable power source, such as a battery. These devices generally require the cannabis oil to flow easily from the cartridge to the heating element to enable vaporization. However, when extracted and refined from the plant material, cannabis oil is very viscous and does not easily flow. Therefore, in a practice borrowed from the e-cigarette industry, many cannabis oil manufacturers combine the oil with thinning agents to improve flow.
Within the context of e-cigarette use and its related health effects, studies have shown that many of the toxic chemicals found in e-cigarette aerosols are produced by the thermal decomposition of thinning agents. Researchers have primarily examined propylene glycol (PG), a petroleum-based liquid, and vegetable glycerin (also called glycerol; VG), a sugar derived from plant oils, as these are the thinning agents that are the most commonly used in the e-cigarette industry. Both of these agents are generally recognized as safe by the Food and Drug Administration (FDA) for use in food, and both are commonly used in foods, pharmaceuticals, and cosmetics. However, research shows that these substances may not be safe to use when they are inhaled as a vapor: When heated to temperatures that are commonly reached by e-cigarette devices, PG and VG produce aerosols that contain carbonyls such as formaldehyde, acetaldehyde, and acrolein.25–33 Although studies show that e-cigarettes generally produce carcinogenic compounds in amounts that are lower than traditional cigarettes, increased vaporization temperatures and some characteristics of the vaporization devices (e.g., type of heating element) can result in carbonyl production that exceeds that of cigarette use.27
The production and inhalation of compounds produced by heated thinning agents may be problematic, as these compounds pose potential health risks. The International Agency for Research of Cancer (IARC) classifies formaldehyde as a Group 1 Agent, which is a compound that is known to be carcinogenic.34 California Proposition 65 also identifies formaldehyde as a known cancer-causing agent.35 The American Cancer Society notes that the inhalation of formaldehyde can cause health effects such as watery, burning eyes, burning of the nose and throat, coughing, wheezing, and nausea.36 Several studies also show an association between formaldehyde exposure and increased incidence of myeloid leukemia37–40 and nasopharyngeal cancer.39
The IARC classifies acetaldehyde as a Group 2B Agent, which is possibly carcinogenic to humans34 and similar to formaldehyde, California Proposition 65 identifies acetaldehyde as a known cancer-causing agent.35 Inhalation of acetaldehyde can cause irritation of the nose, throat, and lungs,41 and in animal models it has been shown to cause cancer of the nasal mucosa and larynx.42 Acetaldehyde exposure poses additional risks to individuals who are unable to metabolize acetaldehyde due to a variant copy of the ALDH2 gene, such as facial flushing, dermatitis, respiratory conditions such as rhinitis and the exacerbation of asthma bronchoconstriction, and increased risk of cancer of the head, neck, and esophagus.43
Although not identified as carcinogenic, the U.S. Environmental Protection Agency has identified acrolein as a substance that, at low levels, causes irritation of the eyes and throat and can damage the lining of the lungs.44Abundantly found in cigarette smoke, studies also show that acrolein causes DNA damage and inhibits DNA repair, which suggests that it is a major determinant of lung cancer and lung carcinogenesis.45,46
Given the increased incidence of vaporizing cannabis oil, it is important to determine the potential health risks that are associated with inhaling compounds produced by the thermal decomposition of cannabis oil thinning agents. Research focused on the effects of e-cigarette use clearly demonstrates the potential dangers of inhaling vaporized PG and VG. However, these findings may not generalize to the vaporization of cannabis oil for two reasons. First, cannabis oil and e-cigarette liquids may not vaporize at comparable temperatures. Reconciliation with findings from e-cigarette research is challenging, as researchers have generally measured the power of vaporizing devices in watts or volts rather than temperature. However, in one study that measured device temperature, Geiss et al.26 found that 20 W resulted in significant carbonyl production from PG and VG, which corresponded to temperatures from 225°C to 325°C. The chemical compounds in cannabis, called cannabinoids, vaporize at temperatures ranging from 157°C to 220°C,47 with combustion beginning at 230°C.21 Therefore, cannabis oil should be heated to a temperature above 220°C to achieve maximal cannabinoid vaporization but no greater than 230°C to avoid the potential harmful effects of combustion. In the present study, we examined thinning agent aerosols for the presence of carcinogenic compounds when heated at this maximal temperature of cannabis vaporization (230°C).
Second, although carbonyl production from vaporized PG and VG is well documented, less is known about polyethylene glycol 400 (PEG 400) and medium chain triglycerides (MCT), two agents that, in addition to PG and VG, are commonly used in the cannabis industry. PEG 400 is a petroleum-derived compound that is commonly used in the pharmaceutical industry, and MCT is a fatty acid derived from coconut or palm oil that is often ingested as food or as a nutritional supplement. Similar to PG and VG, both PEG 400 and MCT are generally recognized as safe for use in food by the FDA; however, the potential health effects of vaporizing these products have not been extensively examined. To our knowledge, Kosmider et al.31 have conducted the only study that has included an examination of PEG 400. Although they found that PEG 400 did not produce any carcinogenic compounds, only one e-cigarette solution containing PEG 400 was tested. MCT has not yet been tested with regard to its use as a vaporized thinning agent. In addition to PG and VG, in the present study, we examined carbonyl production from the thermal decomposition of PEG 400 and MCT.
MATERIALS AND METHODS
The thinning agents were tested in a smoking laboratory. To generate the samples for carbonyl testing, an Aspire Atlantis 2 tank was filled with the thinning agent being tested and coupled to an Evolv DNA 200 vaporizer controller containing a nickel coil. The agents were vaporized at 230°C by using a KC Automation KC-5 analytical smoking machine. Each agent was vaporized in 3 blocks of 25 puffs, for a total of 75 puffs per agent. Because standardized parameters for cannabis vaporization experiments have not yet been determined, in the present study, we adopted testing procedures from e-cigarette laboratory experiment standards: Puffs were taken every 30 sec, each for a duration of 4 sec and a volume of 55 mL, by using a square wave profile.48 All puffs were conducted with the tank oriented in a horizontal position. The devices were weighed both before and after each block of 25 puffs and were allowed to rest for at least 10 min between blocks.
Procedures for the determination of formaldehyde, acetaldehyde, and acrolein were based on the high-performance liquid chromatography carbonyl compound analysis method for mainstream cigarette smoke by CORESTA.49 Aerosol samples were collected in 35 mL of 2,4-dinitrophenylhydazine (DNPH) trapping solution. A 4 mL aliquot of the impinger trapping solution was removed and quenched with 0.2 mL of pyridine. Analyses were performed by using an Agilent Model 1100 High Performance Liquid Chromatograph that was equipped with an Agilent Model 1100 Ultraviolet Detector operating at 365 nm and a Waters Xterra C18 3.0 × 250 mm column to determine the presence and level of formaldehyde, acetaldehyde, and acrolein for each puff block.
RESULTS
Analysis of variance (ANOVA) was used to make statistical comparisons among thinning agents in their production of carbonyls. Three ANOVAs were conducted: one each with acetaldehyde, acrolein, and formaldehyde as the independent variables. Probability values less than 0.05 served as markers of statistical significance, and hypothesis tests were two sided. SPSS version 23, manufactured by IBM, was used to conduct all analyses.
Carbonyl levels were measured in micrograms per puff block (μg/puff block), resulting in 12 total measurements (3 puff blocks × 4 thinning agents). Descriptive statistics for carbonyl levels produced by each thinning agent are presented in Table 1. PEG 400 produced the greatest levels of formaldehyde and acetaldehyde, followed by PG. VG and MCT produced low levels of formaldehyde and acetaldehyde, including levels that did not reach the limit of quantitation (LOQ) for acetaldehyde (VG only) and formaldehyde (both VG and MCT). None of the thinning agents produced acrolein at levels that reached the LOQ.*
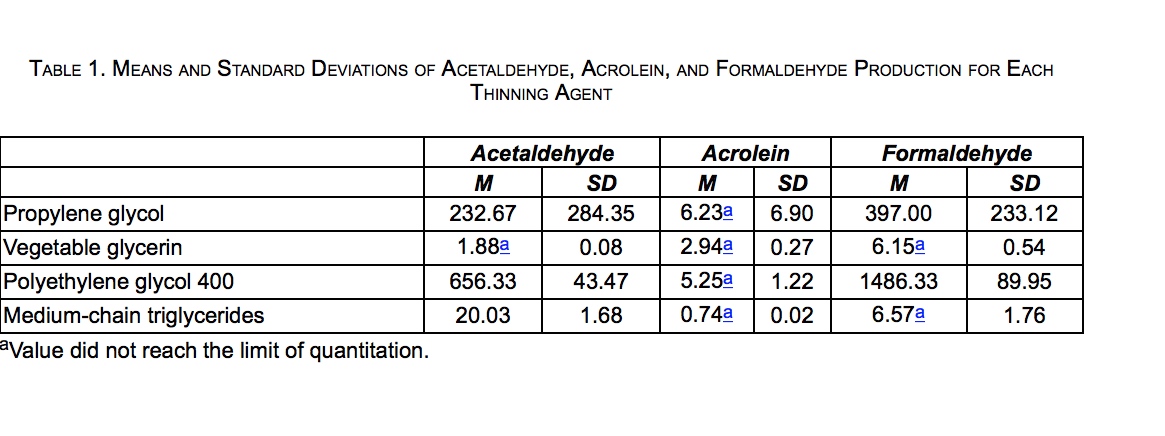
The ANOVA for acetaldehyde revealed a significant effect of thinning agent (p < 0.01, η2 = 0.83). Post hoc Tukey HSD comparisons showed that PEG 400 produced significantly higher levels of acetaldehyde than PG (mean difference = 423.67, p < 0.05, d = 2.58), MCT (mean difference = 636.30, p < 0.01, d = 28.19), and VG (mean difference = 654.45, p < 0.01, d = 30.06). Acetaldehyde production was not significantly different among PG, MCT, and VG.
A similar pattern was found for formaldehyde production. The ANOVA showed a significant overall effect of thinning agent (p < 0.001, η2 = 0.97). Post hoc Tukey HSD comparisons showed that formaldehyde production from PEG 400 was significantly greater than that of PG (mean difference = 1089.33, p < 0.001, d = 6.74), MCT (mean difference = 1479.76, p < 0.001, d = 32.37), and VG (mean difference = 1480.18, p < 0.001, d = 32.71). Formaldehyde production was also significantly greater from PG compared with MCT (mean difference = 390.43, p < 0.05, d = 3.32) and VG (mean difference = 390.85, p < 0.05, d = 3.35). MCT and VG did not produce formaldehyde in amounts that were significantly different from each other.
The omnibus test for the ANOVA for acrolein was not significant (p = 0.294, η2 = 0.36), and thus, it was not examined further.
DISCUSSION
Research shows that many potentially harmful compounds are produced from the thermal decomposition of thinning agents used in e-cigarette devices. Given the increased use of cannabis, particularly vaporized cannabis oil, the goal of the present study was to extend previous research by examining carbonyl formation in cannabis oil thinning agents when heated to a temperature that is appropriate for cannabis vaporization. Specifically, we measured the production of acetaldehyde, formaldehyde, and acrolein when heating PG, VG, PEG 400, and MCT to 230°C.
Compared with the other agents, PEG 400 produced the largest amounts of acetaldehyde and formaldehyde. The amount of formaldehyde was particularly high, with levels that were nearly four times greater than that produced by PG, more than 226 times higher than that produced by MCT, and almost 242 times greater than that produced by VG. Relative to the other agents, PG produced moderate levels of acetaldehyde and formaldehyde. Both VG and MCT produced low levels of acetaldehyde and formaldehyde. All agents produced low levels of acrolein.
To provide a context for exposure to the carbonyls produced by the four agents, we compared the levels of acetaldehyde and formaldehyde to occupational exposure limits defined by the Occupational Safety and Health Administration (OSHA). Leveraging calculations conducted by Gillman et al.,27 the daily OSHA limits for acetaldehyde and formaldehyde are 2,088,000 and 5300 μg, respectively. Given acetaldehyde's greater exposure limit, a cannabis user inhaling the byproducts of heated thinning agents would not be exposed to a significant percentage of their daily limit. For example, one inhalation of PEG 400 heated to 230°C, which produced the greatest amount of acetaldehyde, exposes an individual to 0.00125% of the daily limit. However, for individuals with a variant ALDH2 gene, any exposure to acetaldehyde may cause adverse effects, including an increased risk of UADT cancers.43
Exposure to formaldehyde represents a much greater potential risk. One inhalation of PEG 400 would expose an individual to 1.12% of the daily limit of formaldehyde. Comparatively, smoking one cigarette exposes an individual to 1.42% to 2.35% of the daily limit of formaldehyde.51 Although not as high as PEG 400, one inhalation of PG exposes an individual to 0.30% of the daily limit. In comparison, one inhalation of MCT or VG would result in an exposure of 0.0050% and 0.0046% of the daily limit, respectively. Although in practice only a small amount of PEG 400 or PG is used to dilute cannabis oil (compared with the isolates used in the present study), these results suggest that consumers potentially expose themselves to health risks when using such products, as formaldehyde inhalation has been linked to increased incidence of myeloid leukemia37–40 and nasopharyngeal cancer.39
The results of the present study further substantiate previous research demonstrating carbonyl production from heated PG and VG. However, there is some variability across studies. For example, some studies25,26 show that acetaldehyde is produced primarily by PG, acrolein is produced primarily by VG, and both PG and VG produced formaldehyde; however, others31 (including the present study) show that PG produces acetaldehyde and formaldehyde, VG does not produce elevated levels of any carbonyls, and acrolein is produced by neither PG nor VG. In addition, Kosmider et al.31 did not detect carbonyl production in the single e-cigarette solution tested that contained PEG 400, whereas the present study showed that PEG 400 generated the highest levels of acetaldehyde and formaldehyde.
These inconsistencies may be a function of variability in the temperature reached by vaporization devices across studies, which is dependent on the power supplied to the heating element. For example, in testing several wattages, Geiss et al.26 found that 20 W of power was required for PG and VG to produce significant levels of acrolein and for VG to produce significant levels of formaldehyde. Because 20 W corresponded to temperatures from 225°C to 325°C,26230°C may not have been a temperature that was sufficient to result in acrolein production from PG or VG or formaldehyde production from VG.
Although Kosmider et al.31 also examined the effect of increased power levels on carbonyl formation in thinning agents, the authors did not report the temperatures reached by the device's heating element. Thus, with regard to PEG 400, it is unknown whether temperature differences were what resulted in the inconsistent findings between that study and those of the present study. Furthermore, other factors, such as the type of heating element, also affect carbonyl formation.27 These factors underscore the need for further research on all thinning agents to identify the factors that contribute to increased carbonyl formation.
Some limitations should be considered when interpreting the results of this study. First, limited statistical power may have obscured some potentially large differences in carbonyl formation. For example, PG produced acetaldehyde at levels that were 11.6 and 123.8 times greater than MCT and VG, respectively, and MCT produced acetaldehyde at levels that were 10.7 times greater than VG; however, these differences were not found to be statistically significant. In addition, the ANOVA for acrolein was not statistically significant, despite a large effect size for the omnibus test. Further research with larger samples is needed to adequately ascertain the significance of these differences; however, the results of the present study show that these may be large absolute differences.
Second, thinning agents were tested in isolation. This does not reflect consumer practice, as thinning agents are mixed with cannabis oil for consumption. For two reasons, the results may have differed if a cannabis oil-thinning agent mixture were tested. First, the mixture may have produced a different amount of carcinogenic byproducts than the thinning agents alone. A mixture of two components may have boiling and combustion points that are different from either of the components separately. Thus, vaporizing the mixture may increase or decrease carbonyl production. Second, the botanical and chemical compounds found in cannabis oil may affect carbonyl production during vaporization. Cannabis contains hundreds of cannabinoids, terpenoids, and antioxidants that may affect the oxidation of the thinning agents and inhibit or exacerbate the formation of carcinogenic compounds. Unfortunately, due to federal restrictions, in the present study, we were not able to examine carbonyl production in cannabis oil-thinning agent mixtures. However, we hope that this research serves as a foundation for future work that analyzes carbonyl production when thinning agents are mixed with cannabis oil.
Finally, although acetaldehyde, acrolein, and formaldehyde are the carbonyls that are the most commonly tested for in prior research, thinning agents may produce other potentially harmful compounds. Future work may extend the findings of this study by testing agents for other carbonyls.
CONCLUSIONS
The results of the present study showed that, when heated to 230°C, PEG 400 and PG produce formaldehyde and acetaldehyde (PEG 400 only) at levels that are significantly greater than those produced by MCT and VG. The production of formaldehyde by PEG 400, in particular, may represent a significant health risk, as one inhalation of vaporized PEG 400 isolate may expose an individual to as much as 1.12% of the daily exposure limit, nearly the same exposure as smoking one cigarette. These findings have implications for individuals who vaporize cannabis oil, as cannabis oil that is produced for vaporization is often mixed with PEG 400 or PG, which may result in exposure to harmful carcinogenic compounds and subsequent health risks. More research should be conducted on the potential health concerns of vaporized products as well as long-term studies should be conducted on the actual health effects of vaporizing these products. Patients and policy makers should consider these potential concerns and health effects before use and when drafting legislation that regulates cannabis products.
ACKNOWLEDGMENTS
The authors wish to recognize Gene Gillman, Bryan Tyler, and the staff at Enthalpy Analytical who performed the laboratory testing of the thinning agents investigated in this study. All staff received contractual financial compensation. Randy Taylor Consulting, an organization that provides management services to medical cannabis companies, provided funding for this study. Randy Taylor Consulting had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. Dr. Troutt serves as Medical Director for seven medical cannabis dispensaries in Arizona and as Director of Medical Education for two medical cannabis dispensaries in Nevada. Dr. DiDonato is employed by Randy Taylor Consulting, a management company that provides management services for medical cannabis facilities.
AUTHOR DISCLOSURE STATEMENT
Dr. DiDonato is employed by Randy Taylor Consulting, a management company that provides management services for medical cannabis facilities. Dr. Troutt serves as Medical Director for seven medical cannabis dispensaries in Arizona and as Director of Medical Education for two medical cannabis dispensaries in Nevada.
REFERENCES
1. DS Hasin, TD Saha, BT Kerridge, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015;72:1235–1242.
2. J McCarthy. One in eight U.S. adults say they smoke marijuana. Gallup website, August 8, 2016. Online document at: www.gallup.com/poll/194195/adults-say-smoke-marijuana.aspx Accessed August 24, 2016.
3. S Dutton, J De Pinto, A Salvanto, F Backus. Poll: Support for legal marijuana use reaches all-time high. CBS News website, April 20, 2015. Online document at: www.cbsnews.com/news/poll-support-for-legal-marijuana-use-reaches-all-time-high Accessed July 23, 2016.
4. JM Jones. In U.S., 58% back legal marijuana use. Gallup website, October 21, 2015. Online document at: www.gallup.com/poll/186260/back-legal-marijuana.aspx Accessed July 23, 2016.
5. C Reinarman, H Nunberg, F Lanthier, T Heddleston. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs 2011;43:128–135.
6. WD Troutt, MD DiDonato. Medical cannabis patients in Arizona: Patient characteristics, perceptions, and impressions of medical cannabis legalization. J Psychoactive Drugs 2015;47:259–266.
7. D Moir, WS Rickert, G Levasseur, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 2008;21:494–502.
8. TC Wu, DP Tashkin, B Djahed, JE Rose. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med 1988;318:347–351.
9. RC Callaghan, P Allebeck, A Sidorchuk. Cannabis use and risk of lung cancer: A 40-year cohort study of Swedish men. Eur J Public Health 2014;24:126.
10. YH Huang, ZF Zhang, DP Tashkin, et al. An epidemiological review of marijuana and cancer: An update. Cancer Epidemiol Biomarkers Prev 2015;24:15–31.
11. DP Tashkin. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10:239–247.
12. R Mehra, BA Moore, K Crothers, et al. The association between marijuana smoking and lung cancer. Arch Intern Med 2006;166:1359–1367.
13. MP Martinasek, JB McGrogan, A Maysonet. A systematic review of the respiratory effects of inhalational marijuana. Respir Care 2016;61:1543–1551.
14. J Berthiller, K Straif, M Boniol, et al. Cannabis smoking and risk of lung cancer in men: A pooled analysis of three studies in Maghreb. J Thorac Oncol 2008;3:1398–1403.
15. S Aldington, M Harwood, B Cox, et al. Cannabis use and risk of lung cancer: A case-control study. Eur Respir J2008;31:280–286.
16. S Aldington, M Williams, M Nowitz, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 2007;62:1058–1063.
17. M Earlywine, SS Barnwell. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J2007;4:11.
18. DR Taylor, R Poulton, TE Moffitt, et al. The respiratory effects of cannabis dependence in young adults. Addiction 2000;95:1669–1677.
19. JM Tetrault, K Crothers, BA Moore, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: A systematic review. Arch Intern Med 2007;167:221–228.
20. V Varlet, N Concha-Lozano, A Berthet, et al. Drug vaping applied to cannabis: Is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep 2016;26:25599.
21. DH Gieringer. Cannabis “Vaporization”: A promising strategy for smoke harm reduction. J Cannabis Ther2001;1:153–170.
22. DI Abrams, HP Vizoso, SB Shade, et al. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther 2007;82:52–578.
23. R Baca. Colorado shops sold more than $92 million of pot in February’16. The Cannabist website, April 13, 2016. www.thecannabist.co/2016/04/13/colorado-marijuana-sales-february/51874 Accessed July 12, 2016.
24. R Bingham. Actionable insights on the Washington cannabis market. BDS Analytics website, January 14, 2016. www.bdsanalytics.com/wp-content/uploads/2015/04/1-19-16BDS-Analytics-WASHINGTON-Cannabis-Trends-FINAL.pdf Accessed August 12, 2016.
25. M Sleiman, JM Logue, VN Montesinos, et al. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ Sci Technol 2016;50:9644–9651.
26. O Geiss, I Bianchi, J Barrero-Moreno. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 2016;219:268–277.
27. IG Gillman, KA Kistler, EW Stewart, AR Paolantonio. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 2016;75:58–65.
28. ML Goniewicz, J Knysak, M Gawron, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–139.
29. W Guthery. Emissions of toxic carbonyls in an electronic cigarette. Contrib Tob Res 2016;27:30–37.
30. RP Jensen, W Luo, JF Pankow, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med2015;372:392–394.
31. L Kosmider, A Sobczak, M Fik, et al. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319–1326.
32. R Tayyarah, GA Long. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 2014;70:704–710.
33. S Uchiyama, K Ohta, Y Inaba, N Kunugita. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 2013;29:1219–1222.
34. IARC. Monographs on the evaluation of carcinogenic risks to humans: Agents classified by the IARC monographs, vol. 1-116. International Agency for Research on Cancer website, June 24, 2016. Online document at: http://monographs.iarc.fr/ENG/Classification/index.php Accessed August 1, 2016.
35. OEHHA. Chemicals known to the state to cause cancer or reproductive toxicity. State of California Environmental Protection Agency Office of Environmental Health and Hazard Assessment website, August 5, 2016. Online document at: http://oehha.ca.gov/media/downloads/proposition-65//p65single080516.pdf Accessed August 21, 2016.
36. American Cancer Society. Formaldehyde: What is formaldehyde? American Cancer Society website, May 23, 2014. Online document at: www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplace/formaldehydeAccessed January 9, 2017.
37. M Hauptmann, PA Stewart, JH Lubin, et al. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst 2009;101:1696–1708.
38. M Hauptmann, JH Lubin, PA Stewart, et al. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J Natl Cancer Inst 2003;95:1615–1623.
39. L Beane Freeman, A Blair, JH Lubin, et al. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute Cohort. J Natl Cancer Inst 2009;101:751–761.
40. LE Pinkerton, MJ Hein, LT Stayner. Mortality among a cohort of garment workers exposed to formaldehyde: An update. Occup Environ Med 2004;61:193–200.
41. Center for Disease Control. Occupational Health Guideline for Acetaldehyde. Center for Disease Control website, September 1978. Online document at: www.cdc.gov/niosh/docs/81-123/pdfs/0001.pdf Accessed January 9, 2017.
42. International Agency for Research on Cancer (IARC). Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC Monogr Eval Carcinog Risks Hum 1999;71:319–325.
43. C Chen, JCB Ferreira, ER Gross, D Mochly-Rosen. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol Rev 2014;94:1–34.
44. USEPA. Toxicological review of acrolein. United States Environmental Protection Agency website, May 2003. Online document at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0364tr.pdf Accessed August 21, 2016.
45. Z Feng, W Hu, Y Hu, MS Tang. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA 2006;103:15404–15409.
46. HT Wang, Y Hu, D Tong, et al. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem 2012;287:12379–12386.
47. JM McPartland, EB Russo. Cannabis and cannabis extracts: Greater than the sum of their parts? Cannabis Ther2001;1:103–132.
48. KE Farsalinos, G Romagna, D Tsiapras, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health 2013;10:2500–2514.
49. CORESTA. Recommended Method No. 74: Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. Cooperation Centre for Scientific Research Relative to Tobacco website, July 2014. Online document at: www.coresta.org/determination-selected-carbonyls-mainstream-cigarette-smoke-high-performance-liquid-chromatography Accessed August 1, 2016.
50. RJ Keizer, RS Jansen, H Rosing, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect 2015;3:e00131.
51. ME Counts, MJ Morton, SW Laffoon, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol 2005;41:185–227.
Date: March 29, 2017
Source: The Journal of Alternative and Complementary Medicine.
To cite article:
Troutt William D. and DiDonato Matthew D.. The Journal of Alternative and Complementary Medicine. March 2017, ahead of print. https://doi.org/10.1089/acm.2016.0337
Author information:
William D. Troutt, NMD, and Matthew D. DiDonato, PhD
Medical Marijuana Research Institute, Tempe, AZ.
Address correspondence to:
Matthew D. DiDonato, PhD
Medical Marijuana Research Institute
627 S. 48th Street Suite 100
Tempe, AZ 85281
E-mail: matthew.didonato@gmail.com
ABSTRACT
Objective: Cannabis use has increased in the United States, particularly the use of vaporized cannabis oil, which is often mixed with thinning agents for use in vaporizing devices. E-cigarette research shows that heated thinning agents produce potentially harmful carbonyls; however, similar studies have not been conducted (1) with agents that are commonly used in the cannabis industry and (2) at temperatures that are appropriate for cannabis oil vaporization. The goal of this study was to determine whether thinning agents used in the cannabis industry produce potentially harmful carbonyls when heated to a temperature that is appropriate for cannabis oil vaporization.
Design: Four thinning agents (propylene glycol [PG], vegetable glycerin [VG], polyethylene glycol 400 [PEG 400], and medium chain triglycerides [MCT]) were heated to 230°C and the resulting vapors were tested for acetaldehyde, acrolein, and formaldehyde. Each agent was tested three times.
Setting/Location: Testing was conducted in a smoking laboratory.
Outcome measures: Carbonyl levels were measured in micrograms per puff block.
Results: Analyses showed that PEG 400 produced significantly higher levels of acetaldehyde and formaldehyde than PG, MCT, and VG. Formaldehyde production was also significantly greater in PG compared with MCT and VG. Acrolein production did not differ significantly across the agents.
Conclusions: PG and PEG 400 produced high levels of acetaldehyde and formaldehyde when heated to 230°C. Formaldehyde production from PEG 400 isolate was particularly high, with one inhalation accounting for 1.12% of the daily exposure limit, nearly the same exposure as smoking one cigarette. Because PG and PEG 400 are often mixed with cannabis oil, individuals who vaporize cannabis oil products may risk exposure to harmful formaldehyde levels. Although more research is needed, consumers and policy makers should consider these potential health effects before use and when drafting cannabis-related legislation.
INTRODUCTION
In the twenty years since California became the first state to legalize medical cannabis, an additional 28 states and the District of Columbia have passed laws permitting cannabis use for medicinal purposes, and eight states have legalized adult use. Consequently, cannabis use in the United States has increased significantly. A study sponsored by the National Institute on Alcohol Abuse and Alcoholism estimated that 9.5% of American adults used cannabis in 2013, up from 4.1% in 2002,1 and a recent Gallup poll found that 13% of adults in the United States currently use cannabis.2Over time, it is likely that more adults will use cannabis, as national polls show that 84% of Americans believe that cannabis should be legalized medicinally3 and 58% support national adult use legalization.4
Given this rapid increase in cannabis use, it is important to examine potential medical and health-related issues. Studies show that as much as 86% of medical cannabis consumers rate smoking as the preferred method of cannabis use.5,6 Therefore, one issue concerns the effect of inhaled cannabis on respiratory function and health. Some research shows that cannabis smoke contains carcinogenic compounds that are similar to those of tobacco smoke, with some compounds in greater quantities than those produced by tobacco,7,8 although studies examining the links between cannabis use and lung cancer are inconclusive.9–15 Some frequent cannabis smokers also experience respiratory issues such as coughing, wheezing, increased sputum production, dyspnea, pharyngitis, and exacerbation of asthma.11,16–19
Due to these issues, cannabis vaporization is becoming more widespread as a potentially safer alternative to smoking. Vaporization is the process of heating cannabis to a temperature at which the plant's chemical compounds boil, creating an aerosol that can be inhaled. Because the cannabis is not heated to the point of combustion, fewer carcinogens and irritants are produced. Compared with smoking, vaporization is associated with fewer respiratory issues in cannabis users,17 which some researchers suggest is a result of lower exposure to toxic substances.17,20,21In addition, Abrams et al.22 found that the amount of inhaled carbon monoxide was significantly lower for vaporized cannabis compared with cannabis that was smoked.
Although several cannabis-derived products can be vaporized, cannabis oil is quickly increasing in popularity. In Colorado, for example, the sale of prefilled cannabis oil cartridges (a product that is exclusively vaporized) increased by 163% from February 2015 to February 2016,23 and in Washington State sales doubled from June 2015 to September 2015.24 For the oil to be vaporized and inhaled, cannabis oil cartridges are typically connected to a vaporizing device that contains a heating element and a disposable or rechargeable power source, such as a battery. These devices generally require the cannabis oil to flow easily from the cartridge to the heating element to enable vaporization. However, when extracted and refined from the plant material, cannabis oil is very viscous and does not easily flow. Therefore, in a practice borrowed from the e-cigarette industry, many cannabis oil manufacturers combine the oil with thinning agents to improve flow.
Within the context of e-cigarette use and its related health effects, studies have shown that many of the toxic chemicals found in e-cigarette aerosols are produced by the thermal decomposition of thinning agents. Researchers have primarily examined propylene glycol (PG), a petroleum-based liquid, and vegetable glycerin (also called glycerol; VG), a sugar derived from plant oils, as these are the thinning agents that are the most commonly used in the e-cigarette industry. Both of these agents are generally recognized as safe by the Food and Drug Administration (FDA) for use in food, and both are commonly used in foods, pharmaceuticals, and cosmetics. However, research shows that these substances may not be safe to use when they are inhaled as a vapor: When heated to temperatures that are commonly reached by e-cigarette devices, PG and VG produce aerosols that contain carbonyls such as formaldehyde, acetaldehyde, and acrolein.25–33 Although studies show that e-cigarettes generally produce carcinogenic compounds in amounts that are lower than traditional cigarettes, increased vaporization temperatures and some characteristics of the vaporization devices (e.g., type of heating element) can result in carbonyl production that exceeds that of cigarette use.27
The production and inhalation of compounds produced by heated thinning agents may be problematic, as these compounds pose potential health risks. The International Agency for Research of Cancer (IARC) classifies formaldehyde as a Group 1 Agent, which is a compound that is known to be carcinogenic.34 California Proposition 65 also identifies formaldehyde as a known cancer-causing agent.35 The American Cancer Society notes that the inhalation of formaldehyde can cause health effects such as watery, burning eyes, burning of the nose and throat, coughing, wheezing, and nausea.36 Several studies also show an association between formaldehyde exposure and increased incidence of myeloid leukemia37–40 and nasopharyngeal cancer.39
The IARC classifies acetaldehyde as a Group 2B Agent, which is possibly carcinogenic to humans34 and similar to formaldehyde, California Proposition 65 identifies acetaldehyde as a known cancer-causing agent.35 Inhalation of acetaldehyde can cause irritation of the nose, throat, and lungs,41 and in animal models it has been shown to cause cancer of the nasal mucosa and larynx.42 Acetaldehyde exposure poses additional risks to individuals who are unable to metabolize acetaldehyde due to a variant copy of the ALDH2 gene, such as facial flushing, dermatitis, respiratory conditions such as rhinitis and the exacerbation of asthma bronchoconstriction, and increased risk of cancer of the head, neck, and esophagus.43
Although not identified as carcinogenic, the U.S. Environmental Protection Agency has identified acrolein as a substance that, at low levels, causes irritation of the eyes and throat and can damage the lining of the lungs.44Abundantly found in cigarette smoke, studies also show that acrolein causes DNA damage and inhibits DNA repair, which suggests that it is a major determinant of lung cancer and lung carcinogenesis.45,46
Given the increased incidence of vaporizing cannabis oil, it is important to determine the potential health risks that are associated with inhaling compounds produced by the thermal decomposition of cannabis oil thinning agents. Research focused on the effects of e-cigarette use clearly demonstrates the potential dangers of inhaling vaporized PG and VG. However, these findings may not generalize to the vaporization of cannabis oil for two reasons. First, cannabis oil and e-cigarette liquids may not vaporize at comparable temperatures. Reconciliation with findings from e-cigarette research is challenging, as researchers have generally measured the power of vaporizing devices in watts or volts rather than temperature. However, in one study that measured device temperature, Geiss et al.26 found that 20 W resulted in significant carbonyl production from PG and VG, which corresponded to temperatures from 225°C to 325°C. The chemical compounds in cannabis, called cannabinoids, vaporize at temperatures ranging from 157°C to 220°C,47 with combustion beginning at 230°C.21 Therefore, cannabis oil should be heated to a temperature above 220°C to achieve maximal cannabinoid vaporization but no greater than 230°C to avoid the potential harmful effects of combustion. In the present study, we examined thinning agent aerosols for the presence of carcinogenic compounds when heated at this maximal temperature of cannabis vaporization (230°C).
Second, although carbonyl production from vaporized PG and VG is well documented, less is known about polyethylene glycol 400 (PEG 400) and medium chain triglycerides (MCT), two agents that, in addition to PG and VG, are commonly used in the cannabis industry. PEG 400 is a petroleum-derived compound that is commonly used in the pharmaceutical industry, and MCT is a fatty acid derived from coconut or palm oil that is often ingested as food or as a nutritional supplement. Similar to PG and VG, both PEG 400 and MCT are generally recognized as safe for use in food by the FDA; however, the potential health effects of vaporizing these products have not been extensively examined. To our knowledge, Kosmider et al.31 have conducted the only study that has included an examination of PEG 400. Although they found that PEG 400 did not produce any carcinogenic compounds, only one e-cigarette solution containing PEG 400 was tested. MCT has not yet been tested with regard to its use as a vaporized thinning agent. In addition to PG and VG, in the present study, we examined carbonyl production from the thermal decomposition of PEG 400 and MCT.
MATERIALS AND METHODS
The thinning agents were tested in a smoking laboratory. To generate the samples for carbonyl testing, an Aspire Atlantis 2 tank was filled with the thinning agent being tested and coupled to an Evolv DNA 200 vaporizer controller containing a nickel coil. The agents were vaporized at 230°C by using a KC Automation KC-5 analytical smoking machine. Each agent was vaporized in 3 blocks of 25 puffs, for a total of 75 puffs per agent. Because standardized parameters for cannabis vaporization experiments have not yet been determined, in the present study, we adopted testing procedures from e-cigarette laboratory experiment standards: Puffs were taken every 30 sec, each for a duration of 4 sec and a volume of 55 mL, by using a square wave profile.48 All puffs were conducted with the tank oriented in a horizontal position. The devices were weighed both before and after each block of 25 puffs and were allowed to rest for at least 10 min between blocks.
Procedures for the determination of formaldehyde, acetaldehyde, and acrolein were based on the high-performance liquid chromatography carbonyl compound analysis method for mainstream cigarette smoke by CORESTA.49 Aerosol samples were collected in 35 mL of 2,4-dinitrophenylhydazine (DNPH) trapping solution. A 4 mL aliquot of the impinger trapping solution was removed and quenched with 0.2 mL of pyridine. Analyses were performed by using an Agilent Model 1100 High Performance Liquid Chromatograph that was equipped with an Agilent Model 1100 Ultraviolet Detector operating at 365 nm and a Waters Xterra C18 3.0 × 250 mm column to determine the presence and level of formaldehyde, acetaldehyde, and acrolein for each puff block.
RESULTS
Analysis of variance (ANOVA) was used to make statistical comparisons among thinning agents in their production of carbonyls. Three ANOVAs were conducted: one each with acetaldehyde, acrolein, and formaldehyde as the independent variables. Probability values less than 0.05 served as markers of statistical significance, and hypothesis tests were two sided. SPSS version 23, manufactured by IBM, was used to conduct all analyses.
Carbonyl levels were measured in micrograms per puff block (μg/puff block), resulting in 12 total measurements (3 puff blocks × 4 thinning agents). Descriptive statistics for carbonyl levels produced by each thinning agent are presented in Table 1. PEG 400 produced the greatest levels of formaldehyde and acetaldehyde, followed by PG. VG and MCT produced low levels of formaldehyde and acetaldehyde, including levels that did not reach the limit of quantitation (LOQ) for acetaldehyde (VG only) and formaldehyde (both VG and MCT). None of the thinning agents produced acrolein at levels that reached the LOQ.*

The ANOVA for acetaldehyde revealed a significant effect of thinning agent (p < 0.01, η2 = 0.83). Post hoc Tukey HSD comparisons showed that PEG 400 produced significantly higher levels of acetaldehyde than PG (mean difference = 423.67, p < 0.05, d = 2.58), MCT (mean difference = 636.30, p < 0.01, d = 28.19), and VG (mean difference = 654.45, p < 0.01, d = 30.06). Acetaldehyde production was not significantly different among PG, MCT, and VG.
A similar pattern was found for formaldehyde production. The ANOVA showed a significant overall effect of thinning agent (p < 0.001, η2 = 0.97). Post hoc Tukey HSD comparisons showed that formaldehyde production from PEG 400 was significantly greater than that of PG (mean difference = 1089.33, p < 0.001, d = 6.74), MCT (mean difference = 1479.76, p < 0.001, d = 32.37), and VG (mean difference = 1480.18, p < 0.001, d = 32.71). Formaldehyde production was also significantly greater from PG compared with MCT (mean difference = 390.43, p < 0.05, d = 3.32) and VG (mean difference = 390.85, p < 0.05, d = 3.35). MCT and VG did not produce formaldehyde in amounts that were significantly different from each other.
The omnibus test for the ANOVA for acrolein was not significant (p = 0.294, η2 = 0.36), and thus, it was not examined further.
DISCUSSION
Research shows that many potentially harmful compounds are produced from the thermal decomposition of thinning agents used in e-cigarette devices. Given the increased use of cannabis, particularly vaporized cannabis oil, the goal of the present study was to extend previous research by examining carbonyl formation in cannabis oil thinning agents when heated to a temperature that is appropriate for cannabis vaporization. Specifically, we measured the production of acetaldehyde, formaldehyde, and acrolein when heating PG, VG, PEG 400, and MCT to 230°C.
Compared with the other agents, PEG 400 produced the largest amounts of acetaldehyde and formaldehyde. The amount of formaldehyde was particularly high, with levels that were nearly four times greater than that produced by PG, more than 226 times higher than that produced by MCT, and almost 242 times greater than that produced by VG. Relative to the other agents, PG produced moderate levels of acetaldehyde and formaldehyde. Both VG and MCT produced low levels of acetaldehyde and formaldehyde. All agents produced low levels of acrolein.
To provide a context for exposure to the carbonyls produced by the four agents, we compared the levels of acetaldehyde and formaldehyde to occupational exposure limits defined by the Occupational Safety and Health Administration (OSHA). Leveraging calculations conducted by Gillman et al.,27 the daily OSHA limits for acetaldehyde and formaldehyde are 2,088,000 and 5300 μg, respectively. Given acetaldehyde's greater exposure limit, a cannabis user inhaling the byproducts of heated thinning agents would not be exposed to a significant percentage of their daily limit. For example, one inhalation of PEG 400 heated to 230°C, which produced the greatest amount of acetaldehyde, exposes an individual to 0.00125% of the daily limit. However, for individuals with a variant ALDH2 gene, any exposure to acetaldehyde may cause adverse effects, including an increased risk of UADT cancers.43
Exposure to formaldehyde represents a much greater potential risk. One inhalation of PEG 400 would expose an individual to 1.12% of the daily limit of formaldehyde. Comparatively, smoking one cigarette exposes an individual to 1.42% to 2.35% of the daily limit of formaldehyde.51 Although not as high as PEG 400, one inhalation of PG exposes an individual to 0.30% of the daily limit. In comparison, one inhalation of MCT or VG would result in an exposure of 0.0050% and 0.0046% of the daily limit, respectively. Although in practice only a small amount of PEG 400 or PG is used to dilute cannabis oil (compared with the isolates used in the present study), these results suggest that consumers potentially expose themselves to health risks when using such products, as formaldehyde inhalation has been linked to increased incidence of myeloid leukemia37–40 and nasopharyngeal cancer.39
The results of the present study further substantiate previous research demonstrating carbonyl production from heated PG and VG. However, there is some variability across studies. For example, some studies25,26 show that acetaldehyde is produced primarily by PG, acrolein is produced primarily by VG, and both PG and VG produced formaldehyde; however, others31 (including the present study) show that PG produces acetaldehyde and formaldehyde, VG does not produce elevated levels of any carbonyls, and acrolein is produced by neither PG nor VG. In addition, Kosmider et al.31 did not detect carbonyl production in the single e-cigarette solution tested that contained PEG 400, whereas the present study showed that PEG 400 generated the highest levels of acetaldehyde and formaldehyde.
These inconsistencies may be a function of variability in the temperature reached by vaporization devices across studies, which is dependent on the power supplied to the heating element. For example, in testing several wattages, Geiss et al.26 found that 20 W of power was required for PG and VG to produce significant levels of acrolein and for VG to produce significant levels of formaldehyde. Because 20 W corresponded to temperatures from 225°C to 325°C,26230°C may not have been a temperature that was sufficient to result in acrolein production from PG or VG or formaldehyde production from VG.
Although Kosmider et al.31 also examined the effect of increased power levels on carbonyl formation in thinning agents, the authors did not report the temperatures reached by the device's heating element. Thus, with regard to PEG 400, it is unknown whether temperature differences were what resulted in the inconsistent findings between that study and those of the present study. Furthermore, other factors, such as the type of heating element, also affect carbonyl formation.27 These factors underscore the need for further research on all thinning agents to identify the factors that contribute to increased carbonyl formation.
Some limitations should be considered when interpreting the results of this study. First, limited statistical power may have obscured some potentially large differences in carbonyl formation. For example, PG produced acetaldehyde at levels that were 11.6 and 123.8 times greater than MCT and VG, respectively, and MCT produced acetaldehyde at levels that were 10.7 times greater than VG; however, these differences were not found to be statistically significant. In addition, the ANOVA for acrolein was not statistically significant, despite a large effect size for the omnibus test. Further research with larger samples is needed to adequately ascertain the significance of these differences; however, the results of the present study show that these may be large absolute differences.
Second, thinning agents were tested in isolation. This does not reflect consumer practice, as thinning agents are mixed with cannabis oil for consumption. For two reasons, the results may have differed if a cannabis oil-thinning agent mixture were tested. First, the mixture may have produced a different amount of carcinogenic byproducts than the thinning agents alone. A mixture of two components may have boiling and combustion points that are different from either of the components separately. Thus, vaporizing the mixture may increase or decrease carbonyl production. Second, the botanical and chemical compounds found in cannabis oil may affect carbonyl production during vaporization. Cannabis contains hundreds of cannabinoids, terpenoids, and antioxidants that may affect the oxidation of the thinning agents and inhibit or exacerbate the formation of carcinogenic compounds. Unfortunately, due to federal restrictions, in the present study, we were not able to examine carbonyl production in cannabis oil-thinning agent mixtures. However, we hope that this research serves as a foundation for future work that analyzes carbonyl production when thinning agents are mixed with cannabis oil.
Finally, although acetaldehyde, acrolein, and formaldehyde are the carbonyls that are the most commonly tested for in prior research, thinning agents may produce other potentially harmful compounds. Future work may extend the findings of this study by testing agents for other carbonyls.
CONCLUSIONS
The results of the present study showed that, when heated to 230°C, PEG 400 and PG produce formaldehyde and acetaldehyde (PEG 400 only) at levels that are significantly greater than those produced by MCT and VG. The production of formaldehyde by PEG 400, in particular, may represent a significant health risk, as one inhalation of vaporized PEG 400 isolate may expose an individual to as much as 1.12% of the daily exposure limit, nearly the same exposure as smoking one cigarette. These findings have implications for individuals who vaporize cannabis oil, as cannabis oil that is produced for vaporization is often mixed with PEG 400 or PG, which may result in exposure to harmful carcinogenic compounds and subsequent health risks. More research should be conducted on the potential health concerns of vaporized products as well as long-term studies should be conducted on the actual health effects of vaporizing these products. Patients and policy makers should consider these potential concerns and health effects before use and when drafting legislation that regulates cannabis products.
ACKNOWLEDGMENTS
The authors wish to recognize Gene Gillman, Bryan Tyler, and the staff at Enthalpy Analytical who performed the laboratory testing of the thinning agents investigated in this study. All staff received contractual financial compensation. Randy Taylor Consulting, an organization that provides management services to medical cannabis companies, provided funding for this study. Randy Taylor Consulting had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. Dr. Troutt serves as Medical Director for seven medical cannabis dispensaries in Arizona and as Director of Medical Education for two medical cannabis dispensaries in Nevada. Dr. DiDonato is employed by Randy Taylor Consulting, a management company that provides management services for medical cannabis facilities.
AUTHOR DISCLOSURE STATEMENT
Dr. DiDonato is employed by Randy Taylor Consulting, a management company that provides management services for medical cannabis facilities. Dr. Troutt serves as Medical Director for seven medical cannabis dispensaries in Arizona and as Director of Medical Education for two medical cannabis dispensaries in Nevada.
REFERENCES
1. DS Hasin, TD Saha, BT Kerridge, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015;72:1235–1242.
2. J McCarthy. One in eight U.S. adults say they smoke marijuana. Gallup website, August 8, 2016. Online document at: www.gallup.com/poll/194195/adults-say-smoke-marijuana.aspx Accessed August 24, 2016.
3. S Dutton, J De Pinto, A Salvanto, F Backus. Poll: Support for legal marijuana use reaches all-time high. CBS News website, April 20, 2015. Online document at: www.cbsnews.com/news/poll-support-for-legal-marijuana-use-reaches-all-time-high Accessed July 23, 2016.
4. JM Jones. In U.S., 58% back legal marijuana use. Gallup website, October 21, 2015. Online document at: www.gallup.com/poll/186260/back-legal-marijuana.aspx Accessed July 23, 2016.
5. C Reinarman, H Nunberg, F Lanthier, T Heddleston. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs 2011;43:128–135.
6. WD Troutt, MD DiDonato. Medical cannabis patients in Arizona: Patient characteristics, perceptions, and impressions of medical cannabis legalization. J Psychoactive Drugs 2015;47:259–266.
7. D Moir, WS Rickert, G Levasseur, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 2008;21:494–502.
8. TC Wu, DP Tashkin, B Djahed, JE Rose. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med 1988;318:347–351.
9. RC Callaghan, P Allebeck, A Sidorchuk. Cannabis use and risk of lung cancer: A 40-year cohort study of Swedish men. Eur J Public Health 2014;24:126.
10. YH Huang, ZF Zhang, DP Tashkin, et al. An epidemiological review of marijuana and cancer: An update. Cancer Epidemiol Biomarkers Prev 2015;24:15–31.
11. DP Tashkin. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10:239–247.
12. R Mehra, BA Moore, K Crothers, et al. The association between marijuana smoking and lung cancer. Arch Intern Med 2006;166:1359–1367.
13. MP Martinasek, JB McGrogan, A Maysonet. A systematic review of the respiratory effects of inhalational marijuana. Respir Care 2016;61:1543–1551.
14. J Berthiller, K Straif, M Boniol, et al. Cannabis smoking and risk of lung cancer in men: A pooled analysis of three studies in Maghreb. J Thorac Oncol 2008;3:1398–1403.
15. S Aldington, M Harwood, B Cox, et al. Cannabis use and risk of lung cancer: A case-control study. Eur Respir J2008;31:280–286.
16. S Aldington, M Williams, M Nowitz, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 2007;62:1058–1063.
17. M Earlywine, SS Barnwell. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J2007;4:11.
18. DR Taylor, R Poulton, TE Moffitt, et al. The respiratory effects of cannabis dependence in young adults. Addiction 2000;95:1669–1677.
19. JM Tetrault, K Crothers, BA Moore, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: A systematic review. Arch Intern Med 2007;167:221–228.
20. V Varlet, N Concha-Lozano, A Berthet, et al. Drug vaping applied to cannabis: Is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep 2016;26:25599.
21. DH Gieringer. Cannabis “Vaporization”: A promising strategy for smoke harm reduction. J Cannabis Ther2001;1:153–170.
22. DI Abrams, HP Vizoso, SB Shade, et al. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther 2007;82:52–578.
23. R Baca. Colorado shops sold more than $92 million of pot in February’16. The Cannabist website, April 13, 2016. www.thecannabist.co/2016/04/13/colorado-marijuana-sales-february/51874 Accessed July 12, 2016.
24. R Bingham. Actionable insights on the Washington cannabis market. BDS Analytics website, January 14, 2016. www.bdsanalytics.com/wp-content/uploads/2015/04/1-19-16BDS-Analytics-WASHINGTON-Cannabis-Trends-FINAL.pdf Accessed August 12, 2016.
25. M Sleiman, JM Logue, VN Montesinos, et al. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ Sci Technol 2016;50:9644–9651.
26. O Geiss, I Bianchi, J Barrero-Moreno. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 2016;219:268–277.
27. IG Gillman, KA Kistler, EW Stewart, AR Paolantonio. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 2016;75:58–65.
28. ML Goniewicz, J Knysak, M Gawron, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–139.
29. W Guthery. Emissions of toxic carbonyls in an electronic cigarette. Contrib Tob Res 2016;27:30–37.
30. RP Jensen, W Luo, JF Pankow, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med2015;372:392–394.
31. L Kosmider, A Sobczak, M Fik, et al. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319–1326.
32. R Tayyarah, GA Long. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 2014;70:704–710.
33. S Uchiyama, K Ohta, Y Inaba, N Kunugita. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 2013;29:1219–1222.
34. IARC. Monographs on the evaluation of carcinogenic risks to humans: Agents classified by the IARC monographs, vol. 1-116. International Agency for Research on Cancer website, June 24, 2016. Online document at: http://monographs.iarc.fr/ENG/Classification/index.php Accessed August 1, 2016.
35. OEHHA. Chemicals known to the state to cause cancer or reproductive toxicity. State of California Environmental Protection Agency Office of Environmental Health and Hazard Assessment website, August 5, 2016. Online document at: http://oehha.ca.gov/media/downloads/proposition-65//p65single080516.pdf Accessed August 21, 2016.
36. American Cancer Society. Formaldehyde: What is formaldehyde? American Cancer Society website, May 23, 2014. Online document at: www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplace/formaldehydeAccessed January 9, 2017.
37. M Hauptmann, PA Stewart, JH Lubin, et al. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst 2009;101:1696–1708.
38. M Hauptmann, JH Lubin, PA Stewart, et al. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J Natl Cancer Inst 2003;95:1615–1623.
39. L Beane Freeman, A Blair, JH Lubin, et al. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute Cohort. J Natl Cancer Inst 2009;101:751–761.
40. LE Pinkerton, MJ Hein, LT Stayner. Mortality among a cohort of garment workers exposed to formaldehyde: An update. Occup Environ Med 2004;61:193–200.
41. Center for Disease Control. Occupational Health Guideline for Acetaldehyde. Center for Disease Control website, September 1978. Online document at: www.cdc.gov/niosh/docs/81-123/pdfs/0001.pdf Accessed January 9, 2017.
42. International Agency for Research on Cancer (IARC). Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC Monogr Eval Carcinog Risks Hum 1999;71:319–325.
43. C Chen, JCB Ferreira, ER Gross, D Mochly-Rosen. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol Rev 2014;94:1–34.
44. USEPA. Toxicological review of acrolein. United States Environmental Protection Agency website, May 2003. Online document at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0364tr.pdf Accessed August 21, 2016.
45. Z Feng, W Hu, Y Hu, MS Tang. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA 2006;103:15404–15409.
46. HT Wang, Y Hu, D Tong, et al. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem 2012;287:12379–12386.
47. JM McPartland, EB Russo. Cannabis and cannabis extracts: Greater than the sum of their parts? Cannabis Ther2001;1:103–132.
48. KE Farsalinos, G Romagna, D Tsiapras, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health 2013;10:2500–2514.
49. CORESTA. Recommended Method No. 74: Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. Cooperation Centre for Scientific Research Relative to Tobacco website, July 2014. Online document at: www.coresta.org/determination-selected-carbonyls-mainstream-cigarette-smoke-high-performance-liquid-chromatography Accessed August 1, 2016.
50. RJ Keizer, RS Jansen, H Rosing, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect 2015;3:e00131.
51. ME Counts, MJ Morton, SW Laffoon, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol 2005;41:185–227.