RCT CONFIRMS THAT CANNABINOIDS HELP ADULTS WITH ADHD
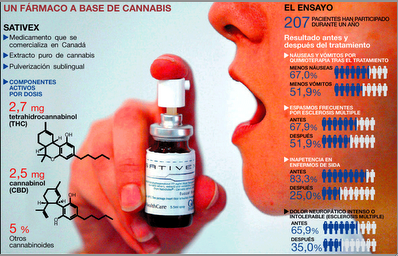
“Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial,” by R.E. Cooper and colleagues at Kings College London has been e-published in European Neuropsychopharmacology.
“Adults with ADHD may represent a subgroup of individuals who experience a reduction of symptoms and no cognitive impairments following cannabinoid use,” the abstract concludes. “While not definitive, this study provides preliminary evidence supporting the self-medication theory of cannabis use in ADHD.” (Thanks to Joe. D. Golstrich, MD, for forwarding.)
The trial by Cooper et al was conducted with Sativex, GW’s Oromucosal Spray, which contains a 50-5o mix of THC and CBD. The peer-reviewed results confer credibility on the thousands of cannabis users who say the herb helps them focus, and on physicians who approve use of cannabis in treating ADHD. This is a good example of how GW Pharmaceuticals continues to advance the medical marijuana movement.
Recently, activists have denounced GW for lobbying state legislatures to pass bills that would make Epidiolex —GW’s almost-pure-CBD anticonvulsant— immediately available if and when the FDA shifts it from Schedule 1. (FDA approval for Epidiolex could come any day now. And no, I don’t own stock in the company.)
The activists fear that their legislators will cite the availability of Epidiolex to continue a ban on other CBD formulations. Wrath has been directed at GW —a more vulnerable target than the Prohibitionist legislators. The sad brouhaha was first publicized by Leafly. David Bienenstock, writing a follow-up piece for Vice, asked me for a comment and then didn’t use it. Here it is, a little dusty from the cutting room floor:
The oddest thing about the Leafly exposé is the image of GW Pharmaceuticals trying to sneak something over on us, hiring lobbyists “on the low down.” Geoffrey W. Guy, MD, is a pharmaceutical entrepreneur and has never pretended to be anything else. His stated plan has always been to seek approval from the regulatory authorities —the Home Office in the UK, the FDA in the US—for GW’s cannabis-based medicines. He laid it all out in 1998 at the International Cannabinoid Research Society meeting, and I reported on it in detail in Synapse (the UCSF weekly). Guy got the go-ahead to cultivate cannabis and produce medicine by convincing the Home Office that a compound in the plant called cannabidiol had medicinal effects of its own and could negate the psychoactivity of THC, and that cannabinoids could be ingested by means other than smoking.
The first issue of O’Shaughnessy’s (Summer 2003) includes a piece describing GW’s progress and so does every issue since. It was GW providing plant extracts to scientists that broke the NIDA monopoly on cannabinoid research. My first goal for Project CBD was to bring US physicians and growers up to speed on what GW had learned.
When I filled my wife in just now she said, “Do these people know that without Geoffrey Guy there would be no CBD?” The woman tends to exaggerate, but her aim is true.
In a report for O’Shaughnessy’s on the 2004 meeting of the International Cannabinoid Research Society, a renowned scientist writing as “Dr. X” was struck by “All the posters on CBD! What a difference a decade makes. I presented a poster about CBD in 1994 and mine was the only mention of CBD at the conference. In 2004 we heard a dozen. The difference between 1994 and 2004? GW Pharmaceuticals sparking the interest and supplying the drug to researchers.”
O’S also published “The Pharmaceuticalization of Marijuana” by Lester Grinspoon, warning that GW’s pursuit of FDA approval would complicate the political movement against Prohibition. “The Ballad of Grinspoon and Guy” laments the tension between the two approaches.
Doctors in the Society of Cannabis Clinicians —doctors who believe their patients— have known all along that cannabis can alleviate symptoms of ADHD. Drs. Tom O’Connell and Claudia Jensen were among the first to publicize this application.

“Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial,” by R.E. Cooper and colleagues at Kings College London has been e-published in European Neuropsychopharmacology.
“Adults with ADHD may represent a subgroup of individuals who experience a reduction of symptoms and no cognitive impairments following cannabinoid use,” the abstract concludes. “While not definitive, this study provides preliminary evidence supporting the self-medication theory of cannabis use in ADHD.” (Thanks to Joe. D. Golstrich, MD, for forwarding.)
The trial by Cooper et al was conducted with Sativex, GW’s Oromucosal Spray, which contains a 50-5o mix of THC and CBD. The peer-reviewed results confer credibility on the thousands of cannabis users who say the herb helps them focus, and on physicians who approve use of cannabis in treating ADHD. This is a good example of how GW Pharmaceuticals continues to advance the medical marijuana movement.
Recently, activists have denounced GW for lobbying state legislatures to pass bills that would make Epidiolex —GW’s almost-pure-CBD anticonvulsant— immediately available if and when the FDA shifts it from Schedule 1. (FDA approval for Epidiolex could come any day now. And no, I don’t own stock in the company.)
The activists fear that their legislators will cite the availability of Epidiolex to continue a ban on other CBD formulations. Wrath has been directed at GW —a more vulnerable target than the Prohibitionist legislators. The sad brouhaha was first publicized by Leafly. David Bienenstock, writing a follow-up piece for Vice, asked me for a comment and then didn’t use it. Here it is, a little dusty from the cutting room floor:
The oddest thing about the Leafly exposé is the image of GW Pharmaceuticals trying to sneak something over on us, hiring lobbyists “on the low down.” Geoffrey W. Guy, MD, is a pharmaceutical entrepreneur and has never pretended to be anything else. His stated plan has always been to seek approval from the regulatory authorities —the Home Office in the UK, the FDA in the US—for GW’s cannabis-based medicines. He laid it all out in 1998 at the International Cannabinoid Research Society meeting, and I reported on it in detail in Synapse (the UCSF weekly). Guy got the go-ahead to cultivate cannabis and produce medicine by convincing the Home Office that a compound in the plant called cannabidiol had medicinal effects of its own and could negate the psychoactivity of THC, and that cannabinoids could be ingested by means other than smoking.
The first issue of O’Shaughnessy’s (Summer 2003) includes a piece describing GW’s progress and so does every issue since. It was GW providing plant extracts to scientists that broke the NIDA monopoly on cannabinoid research. My first goal for Project CBD was to bring US physicians and growers up to speed on what GW had learned.
When I filled my wife in just now she said, “Do these people know that without Geoffrey Guy there would be no CBD?” The woman tends to exaggerate, but her aim is true.
In a report for O’Shaughnessy’s on the 2004 meeting of the International Cannabinoid Research Society, a renowned scientist writing as “Dr. X” was struck by “All the posters on CBD! What a difference a decade makes. I presented a poster about CBD in 1994 and mine was the only mention of CBD at the conference. In 2004 we heard a dozen. The difference between 1994 and 2004? GW Pharmaceuticals sparking the interest and supplying the drug to researchers.”
O’S also published “The Pharmaceuticalization of Marijuana” by Lester Grinspoon, warning that GW’s pursuit of FDA approval would complicate the political movement against Prohibition. “The Ballad of Grinspoon and Guy” laments the tension between the two approaches.
Doctors in the Society of Cannabis Clinicians —doctors who believe their patients— have known all along that cannabis can alleviate symptoms of ADHD. Drs. Tom O’Connell and Claudia Jensen were among the first to publicize this application.