|
Sponsored by |
|---|
|
|
|
-
Need help navigating the forum? Find out how to use our features here.
-
Did you know we have lots of smilies for you to use?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Law The Cannabis Chronicles - Misc Cannabis News
- Thread starter CarolKing
- Start date
Baron23
Well-Known Member
Will medical marijuana survive once everyone can buy cannabis?
On Election Day, voters in New Jersey spoke loudly: legalize the sale of cannabis in our state. But as we move toward legalized recreational cannabis, we must not risk the health of medicinal cannabis patients.
New Jersey became the 14th state to allow the sale of medicinal cannabis when the Compassionate Use Medical Marijuana Act was passed in 2010. Right now, more than 80,000 people in New Jersey use cannabis to treat or alleviate symptoms from 17 debilitating conditions — conditions ranging from the pain and nausea of cancer to epilepsy, ALS and glaucoma.
The New Jersey Medical Marijuana Program is enabling people to improve their quality of life, and, in some cases, to ease their end-of-life journey.
hile I am not opposed to the legalization of recreational cannabis for adults, I am deeply concerned about what the change will mean to people who rely on New Jersey’s medicinal program. In state after state, we’ve seen the rise of a recreational market seriously damage the availability of medicinal cannabis.
Some patients may decide to forego the costly doctor’s office visit, and instead, self-treat their condition with recreational use products. Meanwhile, the cannabis strains that many medicinal patients rely on, in particular those with low levels of THC, the psychoactive component of marijuana, often disappear. The rise of recreational markets leads to increases in the overall levels of THC in the cannabis sold in a state. Growers know their market. Selling cannabis with low THC to the recreational market is like trying to sell low-alcohol vodka.
Meanwhile, prices for the specialized edibles favored by medical consumers often skyrocket.
The Associated Press analyzed four states with medicinal markets that legalized recreational cannabis: Oregon, Nevada, Colorado, Alaska. All four saw a drop in medical patients after the broader legalization of cannabis. The analysis found that in Oregon the number of medical-only retail shops fell from 400 to two. Hundreds of growers who contracted with individual patients to grow specific strains ended that work.
The destruction of medicinal cannabis dispensaries in New Jersey would be tragic. As the founder of the Compassionate Care Foundation in Egg Harbor, I got to know many people who relied on medical cannabis. I spoke with veterans with Post Traumatic Stress Disorder and cancer patients who found cannabis far superior to opioids for pain relief. I met the parents of children with a rare form of epilepsy who told me of their children’s return to a normal life due to low THC cannabis. And as we continue to face an opioid epidemic, I know patients who were able to control their pain with cannabis and finally beat their opioid addictions.
The Murphy administration has been a great supporter of the medicinal marijuana program, and I am eager to work with the administration to create some protections for medicinal cannabis.
I also see the larger issues and prejudices that prevent cannabis from being treated like any other medication. In the United States, cannabis, along with heroin, is labeled a Schedule 1 drug, which means government regulators claim the drug has no currently accepted medical use and a high potential for abuse. That classification hinders the research we need to obtain a greater understanding of cannabis, such as what cannabis strains and dosages work best for certain conditions. With research lacking, physicians are less likely to recommend cannabis. Right now, insurers and payers will not cover cannabis, even for a dying cancer patient.
We created the Cannabis Education and Research Institute (CERI) to advance research and understanding around this ancient botanical, a plant that has clear healing properties. While New Jersey can create some protections for medicinal patients, ultimately we need more research and understanding. I’m urging medicinal cannabis patients to stay in New Jersey Medical Marijuana Program and to stay with their physicians.
Meanwhile, CERI is fighting to end the federal restrictions that hinder research. State legalization alone does not supersede federal restrictions on research. Removing the prohibitive Schedule 1 designation would open the door to more research. If physicians gain more confidence in cannabis — and the drug is proven effective and safe for many conditions — we may see payers step up. That would go a long way to save medical cannabis for the people who need this medication
Congress Will Vote On Federal Marijuana Legalization Next Month, House Leadership Announces
Less than a week after an Election Day in which voters in five states approved ballot measures to legalize marijuana in some form, a top leader in the U.S. House of Representatives announced that the body will hold a floor vote on a bill to end federal cannabis prohibition next month.
While the presidential election has seized national attention, with advocates hoping President-elect Joe Biden will make good on his campaign pledge to pursue modest marijuana reform starting next year, the comprehensive marijuana legalization bill is still in play during the current Congress.
House Majority Leader Steny Hoyer (D-MD) previously announced this summer that the chamber would vote on the Marijuana Opportunity, Reinvestment and Expungement (MORE) Act in September, but that plan was postponed following pushback from certain centrist Democrats who worried about the optics of advancing cannabis reform before passing another coronavirus relief package.
Several of those members were voted out during an election in which voters in red states such as Montana and South Dakota approved cannabis legalization ballot measures, raising questions about the lawmakers’ strategic thinking on the issue.
In any case, Hoyer confirmed on Monday that marijuana legalization is still on the table before the presidential transition and will get a vote in December. That could also raise the pressure on Biden to embrace legalization—a policy he’s declined to adopt despite supermajority support among Democratic voters.
The House will “vote on the MORE Act to decriminalize cannabis and expunge convictions for non-violent cannabis offenses that have prevented many Americans from getting jobs, applying for credit and loans, and accessing opportunities that make it possible to get ahead in our economy,” Hoyer said in a Dear Colleague letter to House members.
The MORE Act, whose lead sponsor is Judiciary Committee Chairman Jerrold Nadler (D-NY), would federally deschedule cannabis, expunge the records of those with prior marijuana convictions and impose a federal five percent tax on sales, revenue from which would be reinvested in communities most impacted by the drug war.
The legislation would also create a pathway for resentencing for those incarcerated for marijuana offenses, as well as protect immigrants from being denied citizenship over cannabis and prevent federal agencies from denying public benefits or security clearances due to its use.
It cleared Nadler’s panel almost a year ago and has been awaiting floor action since.
Justin Strekal, political director of NORML, told Marijuana Moment that the organization looks forward “to working with House leadership to ensure the success of the first ever vote to end marijuana on the floor of a chamber of Congress.”
Even if the bill does pass in the Democratic-controlled chamber, as it’s expected to with some bipartisan support, it remains unlikely that the Senate will follow suit. Majority Leader Mitch McConnell (R-KY) is a champion of the hemp industry but staunchly opposes further marijuana reform.
Even so, a symbolic vote for legalization could send a strong signal to the incoming Biden administration. Vice President-elect Kamala Harris (D-CA) is the lead Senate sponsor of the MORE Act, but she’s indicated she would not proactively push the former vice president to evolve further on cannabis reform.
Given Biden’s former approach to championing punitive anti-drug legislation as a senator and his ongoing obstinance on marijuana legalization at a time when polls show that a clear majority of Americans favor the policy change, there remains some skepticism about his willingness to make good on his campaign promises to prioritize decriminalization or the other reforms he’s discussed.
However, the president-elect has conceded that his work on punitive anti-drug legislation was a “mistake.”
Outside of Congress, Biden could also make moves to advance cannabis reform administratively.
He could, for example, reinstate a version of the Obama-era Justice Department memo that directed federal prosecutors to generally not interfere with state marijuana laws, which was rescinded by the Trump administration in 2018. It is also within the power of the executive branch to reschedule marijuana under the Controlled Substances Act. Biden has pledged to make a move to Schedule II, though that would not achieve many of the changes advocates seek.
The president has the unilateral authority to grant acts of clemency, including pardons and commutations, to people who have been convicted of federal marijuana or other drug offenses. He also gets to appoint an attorney general, drug czar and other officials who will make decisions on how the federal government handles the issue—though many of those officials will be subject to Senate confirmation.
For his part, Rep. Earl Blumenauer (D-OR) told Marijuana Moment in August that “the Biden administration and a Biden Department of Justice would be a constructive player” in advancing legalization.
Read Hoyer’s letter about the lame duck session schedule, including a marijuana vote, below:
Dear Colleague:
As Members of the House prepare to return to session next week, I write to share the busy Floor schedule we have for the remainder of the year.
During the week of November 16-20, much will be happening off the Floor, as Members-elect participate in New Member Orientation and leadership elections take place for both Democrats and Republicans for the 117th Congress. Meanwhile, on the Floor, the House will consider legislation to reauthorize the National Apprenticeship Act and create more than 1 million apprenticeship opportunities by 2025. Advanced by the Education and Labor Committee, this bill streamlines standards for apprenticeship programs, encourages employers to participate, and expands recruitment efforts. It is an important component of the Make It In America plan to grow jobs and opportunities for American workers and businesses and will help us build our economy back better from the crisis created by COVID-19.
Following the Thanksgiving District Work Period, the House is scheduled to be in session Tuesday, December 1 through Friday, December 4 and Monday, December 7 through Thursday, December 10. Members should be prepared to be in session past December 10th if needed to complete our work. Our top priority continues to be enacting legislation to provide relief for those experiencing economic hardship due to COVID-19, and I hope the Senate and the Trump Administration will work with the House to reach agreement on a bill along the lines of what we passed in May and October through the Heroes Act. So far, the Republican-led Senate has ignored this pressing challenge, and it is long past time to act. I hope the Senate will change course and do its job.
Also in December, we will be focused on an omnibus appropriations measure to fund the government for the remainder of FY2021 and prevent a government shutdown on December 11, when the current continuing resolution expires. I also expect the House to consider a conference report on the National Defense Authorization Act to reauthorize key defense programs and ensure that our military has the tools needed to keep our nation safe, as well as a conference report on the Water Resources Development Act. In addition, the House will vote on the MORE Act to decriminalize cannabis and expunge convictions for non-violent cannabis offenses that have prevented many Americans from getting jobs, applying for credit and loans, and accessing opportunities that make it possible to get ahead in our economy.
As we look ahead to the November and December legislative work periods, I hope we can finish the 116th Congress with the same energy and record of achievement that characterized the House over the past two years. We have much work still ahead, and I hope we can move forward together in a spirit of bipartisan cooperation and a determination to finish this year on a strong note, on our way to the very busy 117th Congress that awaits us next year.
Sincerely,
Steny Hoyer”
Senate Committee Urges Hemp THC Rule Change And Includes Numerous Other Marijuana Provisions In Spending Bills
Senate appropriators on Tuesday released several wide-ranging spending bills and related reports for the 2021 fiscal year that include a variety of provisions related to marijuana and hemp.
Perhaps the most consequential new provision from the Senate Appropriations Committee—at least in the short-term—criticizes a proposed hemp rule from the U.S. Department of Agriculture (USDA).
Hemp is defined under federal statute as containing no more than 0.3 percent THC, but the agency proposed a negligence threshold of up to 0.5 percent—and farmers who exceed that limit would have to destoy their crop. The Senate panel is pushing back against that rule, however, urging USDA to reconsider that “arbitrary” policy in light of stakeholder feedback.
The Senate report also says that another USDA provision “creates roadblocks for farmers by requiring an unrealistic timeframe for testing” and asks the agency to “ensure that any final rule is based on science and will ensure a fair and reasonable regulatory framework.” Advocates and stakeholders have made similar arguments since USDA released its interim rule.
Several other cannabis-related provisions that have appeared in prior appropriations bills and reports are back again. Those include measures banning Washington, D.C. from using its own local tax dollars to implement a regulated marijuana market and protecting state medical cannabis programs from federal intervention. Additionally, lawmakers continue to flag barriers to marijuana research caused by federal prohibition.
One new section asks the National Institute on Drug Abuse (NIDA) to add more cannabis-related questions to an annual federal survey of young people. Specifically, lawmakers want to include measures of “consumption of flavored marijuana vapes and marijuana edibles flavored to appeal to adolescents.”
As noted, the spending bill and committee report cannabis provisions largely align with prior years’ spending bill. Beyond the D.C. and medical marijuana sections, senators also called for $5 million in funding for Food and Drug Administration (FDA) research into cannabis and its derivatives, protecting state hemp programs and expanding marijuana studies within the U.S. Department of Health and Human Services (HHS), for instance.
The House advanced its own appropriations bills over the summer that include broader cannabis reform policies, though there is some overlap between the two chambers.
The House versions contain provisions to protect recreational marijuana legalization laws from federal interference, ease cannabis businesses’ access to basic banking services, expand research, oversee the country’s hemp and CBD industries and grant D.C. the ability to legalize recreational sales.
Fiscal year 2021 already began on October 1, so the government has been relying on continuing resolutions (CRs) to keep programs and agencies funded. Hemp advocates celebrated after the president signed a CR in September that extends a 2014 pilot program for the crop until 2021. It was initially set to expire in October.
The current CR in place will end on December 11, meaning that there would be a federal shutdown if lawmakers don’t pass another short-term extension of full-year appropriations package by then.
“By and large, these bills are the product of bipartisan cooperation among members of the committee,” Appropriations Committee Chairman Richard Shelby (R-AL) said of his panel’s new bills in a press release. “Time after time, we have demonstrated our willingness to work together and get the job done. We have before us the opportunity to deliver for the American people once again.”
Hemp advocates celebrated the committee’s pushing USDA on the issue.
“It is clear that YOUR lobbying has been effective, and we are very optimistic that this will make a difference as USDA moves to a Final Rule,” the advocacy group U.S. Hemp Roundtable said in an email blast responding to the THC-related report language.
Below is the full language of each cannabis-related legislative and report provision, separated according to their respective area of policy.
Hemp and CBD
The reports also call on federal agencies to “propose amendments” to resolve concerns about hemp THC limits, study the environmental impacts of hemp cultivation and reject new agricultural user fees, including those imposed on cannabis producers.Hemp.—The Committee is concerned that the interim final rule entitled ‘‘Establishment of a Domestic Hemp Production Program’’ published by the Department of Agriculture in the Federal Register on October 31, 2019 (84 Fed. Reg. 58522) creates roadblocks for farmers by requiring an unrealistic timeframe for testing, the use of Drug Enforcement Administration registered laboratories, the conversion of THCA into THC, a sampling of only flowering tops, and an arbitrary negligence threshold of 0.5 percent. The Committee directs USDA to propose amendments to the interim final rule to ensure that any final rule is based on science and will ensure a fair and reasonable regulatory framework for commercial hemp production in the United States. In addition, the Committee encourages the Secretary to utilize current Agricultural Research Service research to revise the hemp sampling and testing protocols.
Hemp Cultivation Sustainability.—The Committee encourages the Secretary to study the usage and impacts of energy and water in hemp cultivation and controlled environment agriculture and to make recommendations on best practices and standards in both sectors.
Hemp.—The Committee is aware of statements made by the Department acknowledging the eligibility of researchers participating in hemp pilot programs, as defined by Section 7606 of the Agricultural Act of 2014 (Public Law 113–79), to compete for Federal funds awarded by the Department. The Committee directs the Department to work with and inform stakeholders of this eligibility and to support hemp research, as authorized by Section 7606 of the Agricultural Act of 2014 (Public Law 113–79) and Subtitle G of the Agricultural Marketing Act of 1946 (7 U.S.C. 1621–1627, 1635– 1638)
Hemp Germplasm.—The Committee recognizes the increasing demand for hemp for a variety of uses and its growing importance as a crop for U.S. farmers. When the Nation’s hemp germplasm was destroyed in the 1980s, researchers lost access to publicly available germplasm for plant breeding purposes. The Committee directs ARS to establish and maintain a hemp germplasm repository at the Plant Genetics Resources Research Unit and provides no less than the fiscal year 2020 level for this purpose. The Committee also encourages ARS and the Plant Genetics Resources Research Unit to partner with 1890 institutions that have existing institutional capacity on hemp germplasm research, education, and extension capabilities.
Hemp Production Systems.—The Committee recognizes the emerging market potential for U.S. hemp and hemp-based products for a variety of uses. The Committee directs ARS to conduct regionally-driven research, development, and stakeholder engagement to improve agronomic and agro-economic understanding of effectively integrating hemp into existing agricultural cropping, processing, and marketing systems. The Committee provides an increase of $2,000,000 for this purpose. Research, engagement, and technology transfer shall be conducted in strict accordance with all applicable Federal and State authorities and regulations.
Proposed User Fees.—The Committee rejects the Administration’s proposal to administratively implement new user fees to cover the government’s full cost for providing services to certain beneficiaries, including licenses for…domestic hemp production… The Committee strongly believes that USDA should not propose new user fees without taking into account the full impact on farmers, ranchers, and beneficiaries who would be forced to contend with rapid changes in these programs and additional burdensome costs without prior notice.
Cannabis and Cannabis Derivatives.—As previously noted, the Committee provides $5,000,000 to support regulatory activities, including developing policy, and for the FDA to continue to perform its existing regulatory responsibilities, including review of product applications, inspections, enforcement, and targeted research for cannabis-derived substances, such as cannabidiol [CBD]. Within 90 days of enactment of this Act, the FDA shall issue a policy of enforcement discretion with regard to certain products containing CBD meeting the definition of hemp as defined by section 297A of the Agricultural Marketing Act of 1964 (7 U.S.C. 1639). Such enforcement discretion shall be in effect until the FDA establishes a process for stakeholders to notify the FDA of use of CBD in products that include safety studies for intended use per product and makes a determination about such product. In addition, the FDA is encouraged to consider existing and ongoing medical research related to CBD that is being undertaken pursuant to an Investigational New Drug application in the development of a regulatory pathway for CBD in products under the jurisdiction of the FDA and to ensure that any future regulatory activity does not discourage the development of new drugs. The Committee also encourages the FDA to partner with an academic institution to expand sampling studies of CBD products currently on the market.
Hemp-Based Products.—The Committee recognizes the growing interest for U.S. hemp and hemp-based products for a variety of uses and directs FCA to work with the institutions under its jurisdiction to provide access to guaranteed loans for hemp producers and businesses.
The Committee provides a net increase of $38,000,000 for cross cutting, medical product and food safety activities requested in the budget. Included in this funding is…$5,000,000 for Cannabis and Cannabis Derivatives…
Hemp Testing Technology.—The Agriculture Improvement Act of 2018 (Public Law 115–334) removed hemp and its derivatives from the Controlled Substances Act (Public Law 91–513, as amended), and authorized the production, consumption, and sale of hemp and hemp-derived products in the United States. The Act requires random testing to ensure hemp meets the definition under the law of having a delta-9 tetrahydrocannabinol [THC] concentration of less than 0.3 percent. The Committee is aware that DEA has developed field testing kits that can distinguish between hemp and marijuana on-the-spot. The Committee directs the DEA to continue to work to ensure State and local law enforcement have access to this field test technology so they can more efficiently conduct their drug interdiction efforts at the local level. The Committee further directs the DEA to report back to the Committee not later than 180 days after enactment of this act, and not less than every 6 months thereafter, until such time as testing kits are deployed to State and local law enforcement in the field.
Bills funding USDA and the Department of Justice also contain long-standing provisions making clear that federal agencies should not interfere with state hemp research programs.
SEC. 744. None of the funds made available by this Act or any other Act may be used—
(1) in contravention of section 7606 of the Agricultural Act of 2014 (7 U.S.C. 5940), subtitle G of the Agricultural Marketing Act of 1946, or section 10114 of the Agriculture Improvement Act of 2018; or
(2) to prohibit the transportation, processing, sale, or use of hemp, or seeds of such plant, that is grown or cultivated in accordance with subsection section 7606 of the Agricultural Act of 2014 or Subtitle G of the Agricultural Marketing Act of 1946, within or outside the State in which the hemp is grown or cultivated.
SEC. 529. None of the funds made available by this Act may be used in contravention of section 7606 (‘‘Legitimacy of Industrial Hemp Research’’) of the Agricultural Act of 2014 (Public Law 113–79) by the Department of Justice or the Drug Enforcement Administration.
Protecting State Medical Cannabis Laws
The Justice Department bill also includes a rider protecting state medical cannabis programs from federal intervention, though it does not account for South Dakota voters’ recent decision to legalize.SEC. 530. None of the funds made available under this Act to the Department of Justice may be used, with respect to any of the States of Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming, or with respect to the District of Columbia, the Commonwealth of the Northern Mariana Islands, the United States Virgin Islands, Guam, or Puerto Rico, to prevent any of them from implementing their own laws that authorize the use, distribution, possession, or cultivation of medical marijuana.
Marijuana Research
The report for the bill to fund the Department of Health and Human Services contains several passages directing officials to expand marijuana research, including a note that cannabis’s Schedule I status impedes research.Barriers to Research.—The Committee is concerned that restrictions associated with Schedule I of the Controlled Substance Act (Public Law 91–513) effectively limit the amount and type of research that can be conducted on certain Schedule I drugs, especially opioids, marijuana or its component chemicals, and new synthetic drugs and analogs. At a time when as much information as possible is needed about these drugs to find antidotes for their harmful effects, as well as regulatory and other barriers to conducting this research should be addressed.
Flavored THC.—The Committee appreciates the important data collected in the annual NIDA-funded Monitoring the Future [MTF] survey. The Committee recommends the inclusion of questions on consumption of flavored marijuana vapes and marijuana edibles flavored to appeal to adolescents in the annual MTF survey.
Cannabis Research.—The Committee believes that cannabidiol [CBD] and cannabigerol [CBG], compounds found in cannabis, may provide beneficial medicinal effects. However, there is insufficient scientific information about the long-term effects of these compounds. Additional, coordinated research on a national scale could help determine the toxicology and medicinal effects of CBD and CBG. The Committee encourages NIH to consider additional investment in studying the medicinal effects and toxicology of CBD and CBG including clinical trials.
Blocking Washington, D.C. From Legalizing Marijuana Sales
The bill that funds the District of Columbia maintains a current rider banning the city from using its own money to legalize and regulate recreational cannabis sales, whereas the House-passed version of FY2021 legislation proposes repealing the policy.SEC. 809. (a) None of the Federal funds contained in this Act may be used to enact or carry out any law, rule, or regulation to legalize or otherwise reduce penalties associated with the possession, use, or distribution of any schedule I substance under the Controlled Substances Act (21 U.S.C. 801 et seq.) or any tetrahydrocannabinols derivative.
(b) No funds available for obligation or expenditure by the District of Columbia government under any authority may be used to enact any law, rule, or regulation to legalize or otherwise reduce penalties associated with the possession, use, or distribution of any schedule I substance under the Controlled Substances Act (21 U.S.C. 801 et 4 seq.) or any tetrahydrocannabinols derivative for recreational purposes.
Blocking Legalization Advocacy
One bill includes a 1990s-era provision blocking the use of funds for any activity that “promotes the legalization of any drug” classified in Schedule I of the Controlled Substances Act.SEC. 509. (a) None of the funds made available in this Act may be used for any activity that promotes the legalization of any drug or other substance included in schedule I of the schedules of controlled substances established under section 202 of the Controlled Substances Act except for normal and recognized executive-congressional communications.
(b) The limitation in subsection (a) shall not apply when there is significant medical evidence of a therapeutic advantage to the use of such drug or other substance or that federally sponsored clinical trials are being conducted to determine therapeutic advantage.
That provision was the target of a floor amendment last year from Rep. Alexandria Ocasio-Cortez (D-NY), which was defeated resoundingly. The congresswoman framed her proposal as a way to remove barriers to research on the potential medical benefits of psychedelics.
Marijuana Reform Omitted From Biden Transition Plan On Racial Equity Despite Campaign Pledges
Marijuana reform advocates have been looking for signs that an incoming president-elect Joe Biden will make good on his campaign pledge to pursue cannabis policy changes since the former vice president has been projected to win the election. But they didn’t get any such sign in a new racial equity plan his transition team has put forward.
While Biden emphasized on the campaign trail that cannabis decriminalization and expungements would be part of his racial justice agenda, the plan released over the weekend omits any specific mention of marijuana reform.
Many of the proposals are broadly described, however, and it’s possible that a policy like decriminalization could be folded into broader commitments to eliminate “racial disparities and ensuring fair sentences,” for example.
In any case, there’s been some skepticism on the part of advocates that Biden’s stated support for cannabis reform will be matched with administrative action. And although he and Vice President-elect Kamala Harris have repeatedly promised to follow through with decriminalization and expungements if elected, that issue did not make the cut in the new “commitment to uplifting Black and Brown communities.”
The page says Biden is working to “strengthen America’s commitment to justice, and reform our criminal justice system” and lays out other specific promises that were often mentioned on the campaign trail alongside marijuana reform, such as a ban on police chokeholds and creating a national oversight commission to track law enforcement abuses. But cannabis reform is nowhere to be found in the transition team document.
In contrast, a still-live page on Biden’s separate campaign site for his “Plan for Black America” that he rolled out while running for president, includes the pledge to “decriminalize the use of cannabis and automatically expunge all prior cannabis use convictions.”
Lawmakers and advocates frequently cite cannabis reform as a key racial justice measure, pointing out that Black people are significantly more likely to be arrested over marijuana offenses despite the fact that white people consume cannabis at a comparable rate.
A Biden campaign spokesman, when contacted by Marijuana Moment about the omission of the cannabis pledge from the new site, at first argued that it was because the document is focused on economic equity issues. But when it was pointed out that the page also includes several criminal justice-focused proposals such as stopping the “transfer of weapons of war to police forces” and the other related measures, he replied that the omission of marijuana reform didn’t signal a deprioritization of the issue.
“Nothing has changed,” he said, adding that other priorities of the incoming administration, such as LGBT rights, were also not specifically featured in the “Build Back Better” transition site.
Part of advocates’ skepticism about follow through on the issue is related to the fact that Biden played a key role in advancing punitive anti-drug legislation during his time in the Senate and has declined to embrace adult-use legalization despite supermajority support among voters in his own party.
But while the racial equity page doesn’t seem to signal a sense of urgency when it comes to marijuana reform, many advocates are still optimistic that the Biden-Harris election bodes well for the issue overall.
Beyond decriminalization and expungements, Biden favors medical cannabis legalization, modestly rescheduling marijuana under federal law and letting states set their own policies without federal intervention. Harris is the main Senate sponsor of a bill to federally deschedule cannabis, though she has her own history of previously opposing reform.
“To truly achieve racial equity in marijuana policy, President-elect Biden must commit to removing marijuana from the list of federally controlled substances and repairing harms felt by individuals impacted by this country’s racist drug war,” Martiza Perez, director of national affairs at the Drug Policy Alliance, told Marijuana Moment. “Anything less than that is unacceptable and falls short.”
Biden could accomplish that by supporting Harris’s Marijuana Opportunities, Reinvestment and Expungements (MORE) Act to legalize marijuana at the federal level and implement a series of social justice policies. But he’s so far shown no inclination to do so.
House Majority Leader Steny Hoyer (D-MD) announced on Monday that the chamber will hold a floor vote on the bill, which also contains provisions to fund programs to repair some of the harms of the war on drugs, next month. The House was initially expected to do so in September, but it was ultimately postponed after certain centrist Democrats argued the optics of passing the bill would be bad for them before approving another coronavirus relief package.
“Our hope is that as vice president, Senator Harris will continue to champion the MORE Actas she did in the Senate as the bill’s lead sponsor,” Perez said. “This bill would deschedule marijuana at the federal level and provide a path for the resentencing and expungement of marijuana convictions in addition to other social justice components.”
For her part, Harris has indicated that she wouldn’t be proactively pushing Biden to adopt a pro-legalization stance. She did say last month that she has a “deal” with Biden to candidly share her perspective on a range of progressive policies he currently opposes, however, and that includes legalizing cannabis.
The senator also said that month that the administration would have “a commitment to decriminalizing marijuana and expunging the records of people who have been convicted of marijuana offenses.”
That promise is not featured on the transition team’s new racial equity page, however. It states more generally that we “can and must reduce the number of people incarcerated in this country while also reducing crime,” without specifically recognizing the role of the drug war in increasing incarceration rates nationwide.
The page also fails to note another drug policy reform position Biden holds but which advocates are generally opposed to: diverting people away from incarceration for drug possession and forcing them to enroll treatment programs. While reformers don’t want people to go to jail for drugs, of course, they are concerned that mandating treatment through drug courts inappropriately continues to involve the criminal justice system in responding to a health issue.
Meanwhile, advocates have noticed that Biden and Harris haven’t mentioned the cannabis-related campaign pledges since Election Day.
“During the campaign, President-elect Biden and Vice President-elect Harris both pledged to prioritize reforms to our nation’s cannabis policy,” NORML Executive Director Erik Altieri told Marijuana Moment. “They outlined plans that had the intention of ending marijuana possession arrests and getting the federal government off of the backs of states who wish to end their failed prohibitions.”
“Given that marijuana reform efforts were approved in every state they were on the ballot this election, and received more votes than Joe Biden in all of those states, the Biden-Harris administration needs to acknowledge the overwhelming public support these reforms have and move to rapidly champion change at the federal level,” he said. “The results from election night show that we have a mandate from the American people and we intend to make sure that elected officials abide by it.”
Arrests for drug sales, manufacturing and possession amounted to 1,558,862 in 2019—approximately 15 percent of all busts reported to FBI from local and state law enforcement agencies. That’s one new drug case every 20 seconds.
“Our criminal justice system cannot be just unless we root out the racial, gender, and income-based disparities in the system,” the Biden-Harris transition site says. “The system must be focused on redemption and rehabilitation.”
Shortly after becoming the party’s 2020 nominee, the former vice president’s ongoing opposition to recreational legalization is suspected of being at least partly behind the Democratic National Committee platform committee’s vote against adding the reform as a 2020 party plank in July.
So it may be incumbent upon Congress to advance broad legalization after he takes office. And the likelihood of that happening will hinge largely on the makeup of the Senate, which is yet to be determined.
Should Democrats reclaim control of the Senate and keep the House, the chance of advancing reform will be significantly increased. Sen. Chuck Schumer (D-NY), the current top Democrat in the chamber, who would be expected to be installed as the majority leader come January if the party wins enough of the outstanding races, said last month that he will put his own descheduling bill “in play” and that “I think we’ll have a good chance to pass it.”
With a Democratic-controlled Senate and the party still in control of the House, it stands to reason that cannabis reform would move in the 117th Congress, even if the pace of that reform and the administration’s role in promoting it remain uncertain.
That said, if Republicans keep their hold on the Senate, that could seriously hamper reform efforts. Senate Majority Leader Mitch McConnell (R-KY) is an adamant opponent of loosening laws on marijuana, all but ensuring that reform bills would not stand a chance in his chamber even as he has championed hemp legalization. Even modest House-passed legislation focused on banking access for cannabis businesses never received a vote.
“President-elect Biden has both the opportunity and responsibility to call upon lawmakers to advance comprehensive legislation to reform our country’s failed marijuana policies,” Steve Hawkins, executive director at the Marijuana Policy Project, told Marijuana Moment. “While Americans are divided among many issues, legalization is one issue that brings people across the country together. Voters in red states and blue states alike have shown that they support legalization and it’s time for the president and Congress to take real action.”
Outside of Congress, Biden could also make moves to advance cannabis reform administratively.
For example, he could reinstate a version of the Obama-era Justice Department memo that directed federal prosecutors to generally not interfere with state marijuana laws, which was rescinded by the Trump administration in 2018. It is also within the power of the executive branch to reschedule marijuana under the Controlled Substances Act. Biden has pledged to make a move to Schedule II, though that would not achieve many of the changes advocates seek.
The president has the unilateral authority to grant acts of clemency, including pardons and commutations, to people who have been convicted of federal marijuana or other drug offenses. He also gets to appoint an attorney general, drug czar and other officials who will make decisions on how the federal government handles the issue—though many of those officials will be subject to Senate confirmation.
Rep. Earl Blumenauer (D-OR) told Marijuana Moment in August that “the Biden administration and a Biden Department of Justice would be a constructive player” in advancing legalization.
Bcbud
Cannabis enthusiast

America’s Longest Serving Weed Prisoner To Be Released After 31 Years - International Highlife
The man described as the longest-serving inmate for marijuana crimes in the country will be out of a South Florida prison before Christmas. Richard DeLisi was sentenced to 90 years by a Polk County, Florida, judge after his conviction in 1989. He will be released in December, 18 months ahead of...
Bcbud
Cannabis enthusiast

Survey Shows 75% Of Veterans Are Interested In Cannabis
The Fresh Toast - It’s no secret that many veterans are turning to cannabis to combat some of the difficulties they may be facing from serving in the military. - News
Senate Should Take Up Marijuana Banking Bill, GOP Lawmaker Who Could Chair Key Committee Says
A bill to protect banks that service state-legal marijuana businesses could get a fair shot of advancing through the Senate next year, even if Republicans maintain control of the chamber.
While it remains to be seen if Democrats will be able to secure enough seats to reclaim the Senate in two Georgia runoffs in January, the GOP member expected to take helm of the Banking Committee if his party maintains a majority said recently that he’s open to the modest reform.
Asked by American Banker whether he’d be inclined to work with Democrats—particularly Ranking Member Sherrod Brown (D-OH)—on the Secure and Fair Enforcement (SAFE) Banking Act, Sen. Pat Toomey (R-PA) replied in the affirmative.
“I am open to working with my colleagues on how we could enable businesses that are operating legally in their respective states to be able to have ordinary banking services,” he said. “I think that’s something we should work on.”
The would-be chairman made similar remarks to Politico earlier this month, stating that he is “sympathetic to the idea that people who are involved in [the] cannabis industry—in an entirely legal fashion—ought to be able to have ordinary banking services.”
While Toomey has not embraced broad marijuana reforms such as legalization, he did praisea 2015 Obama administration move to ease some restrictions on cannabis research.
It’s worth noting that his latest comments were prompted by the banking-focused publication under the assumption that Brown would push for the legislation in his leadership position. But the Democrat signaled differently in an interview last week, sayingthat when it comes to the broader issue of marijuana, “I just don’t think that’s a major priority compared to so much else.”
Current Banking Chairman Mike Crapo (R-ID) has previously expressed a willingness to put the cannabis banking bill to a vote in his panel, but it has yet to move since the House approved it last year. He said in 2019 that they were “looking to see whether we can thread the needle” on the legislation.
The chairman has called for certain revisions to the proposal, including some that industry stakeholders have described as untenable, such as making it so only marijuana businesses that cap THC content for their products at two percent would be eligible for banking services.
As currently written, the SAFE Banking Act would not impose such restrictions—and the Democratic-controlled House has approved it three times. The first time was as standalone legislation that cleared the chamber on a largely bipartisan basis. The next two times, it advanced as a provision of broad coronavirus relief packages.
Senate Minority Leader Chuck Schumer (D-NY) also filed his own COVID-19 bill last month that contained the marijuana banking language, but that has not advanced.
When the House approved its coronavirus legislation with the SAFE Banking Act attached, it attracted controversy, with multiple Republican lawmakers and White House officialscriticizing its inclusion and arguing that it is not germane to the issue at hand.
Senate Majority Leader Mitch McConnell (R-KY) in particular has been a vocal opponent of the measure, though he’s largely focused his criticism on certain provisions of the SAFE Banking Act that require industry diversity reporting.
Democratic leaders in both chambers, however, have made clear that they’re willing to keep up the fight, and the House even highlighted the diversity component in a summary of its legislation. House Speaker Nancy Pelosi (D-CA) said in July that she agrees that the banking measure is an appropriate component of the bill.
Also in July, bipartisan treasurers from 15 states and one territory sent a letter to congressional leadership, urging the inclusion of the SAFE Banking Act in any COVID-19 legislation that’s sent to the president’s desk. Following GOP attacks on the House proposal, a group of Democratic state treasurers renewed that call.
The fate of the banking bill in the Senate may rest on the outcome of the two runoff elections that will decide which party controls the chamber. But given Toomey’s comments in favor of taking up the issue—and Brown’s dismissive remarks about cannabis reform—it’s not clear which potential chairman might end up moving the bill faster if his party wins a majority.
Baron23
Well-Known Member
New Poll: Seventy-Percent Of Americans Support Marijuana Legalization
Nearly seven in 10 Americans now support legalizing marijuana nationwide, according to a Gallup poll released on Monday.
Overall, 68 percent of respondents said they favor legalizing cannabis for adult use, which is “Gallup's highest reading” since the firm started polling voters on the issue, it said. Last year, the survey found 66 percent support for legalization.
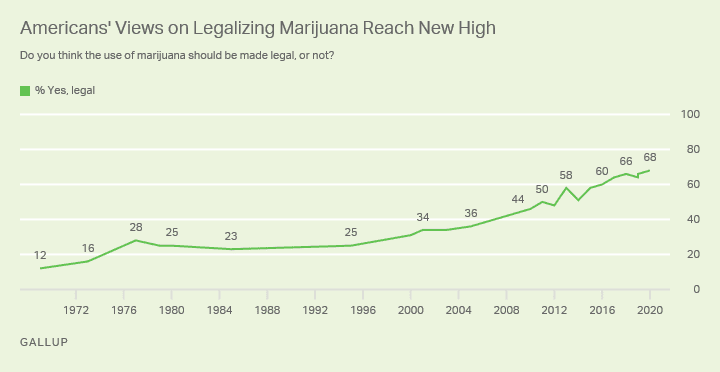
In 1969, only 12 percent of Americans favored legalizing marijuana. Today's level of support is double what it was in 2000.
The new poll shows majority backing for the policy change across all age demographics for the first time. However, support among Republicans dipped slightly compared to last year, from 51 to 48 percent.
Meanwhile, 83 percent of Democrats and 72 percent of independents said they back legalization, which Gallup says is the highest level of support it has ever recorded for those political groups.
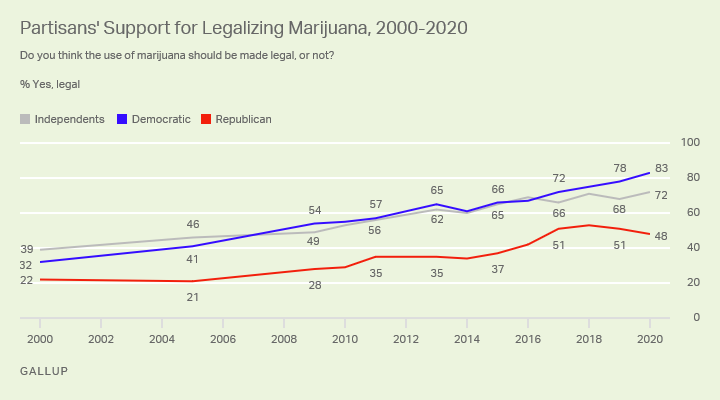
The release of the survey results comes one week after voters in five states approved initiatives to legalize cannabis for medical or recreational purposes. That includes reform wins in traditionally conservative states such as Montana and South Dakota.
“Majorities of most demographic subgroups of Americans support legalizing marijuana, including by gender, age, education and household income,” Gallup, which conducted the survey of 1,035 adults from September 30 to October 15, said.
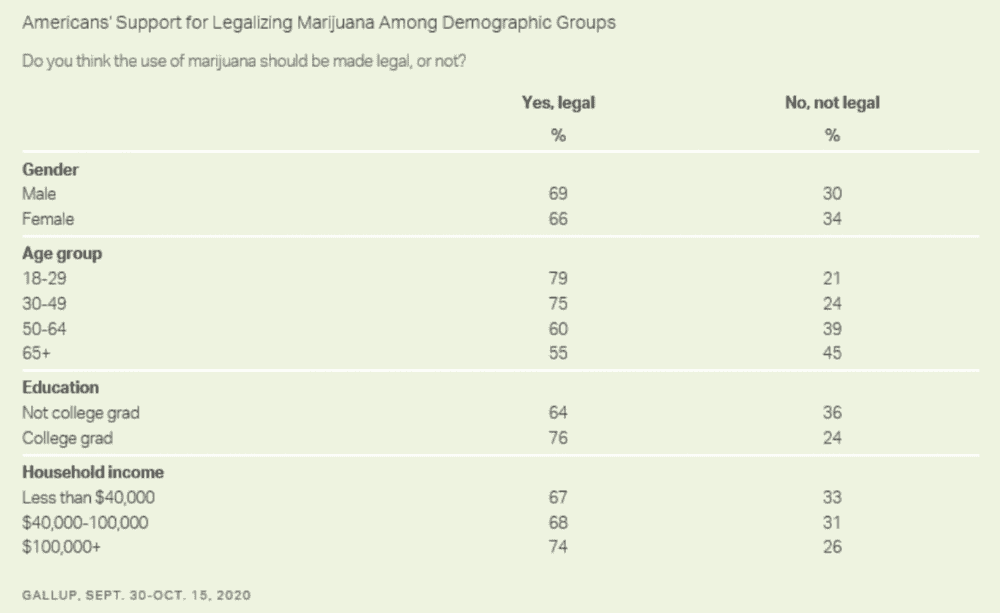
Despite the overwhelming support for the policy change among Democrats, President-elect Joe Biden has so far only backed more modest reforms such as decriminalizing possession and expunging prior cannabis convictions.
Meanwhile, it's not clear why there was a small reduction in support from those who identify as Republican following years of increases. That said, Democratic lawmakers have increasingly attempted to own the issue, which could help explain why fewer conservatives are willing to openly back the policy.
There's also a margin of error of +/- four percentage points in the survey, which could account for the small amount of movement reported among the political demographic.
“Since 2012, when Colorado and Washington became the first states to legalize recreational marijuana, there has been a slow trickle of states that have followed suit,” Gallup said. “Over that period, Americans' support for marijuana legalization has risen 20 points to a record-high 68 percent.”
The firm also referenced a separate survey it conducted earlier this year that showed that about 70 percent of Americans view smoking cannabis to be a morally acceptable activity. That's higher than their views on the morality of issues such as gay relationships, medical testing of animals, the death penalty and abortion.
That said, the new poll found that Americans who more regularly attend religious services are less likely to support legalizing marijuana.
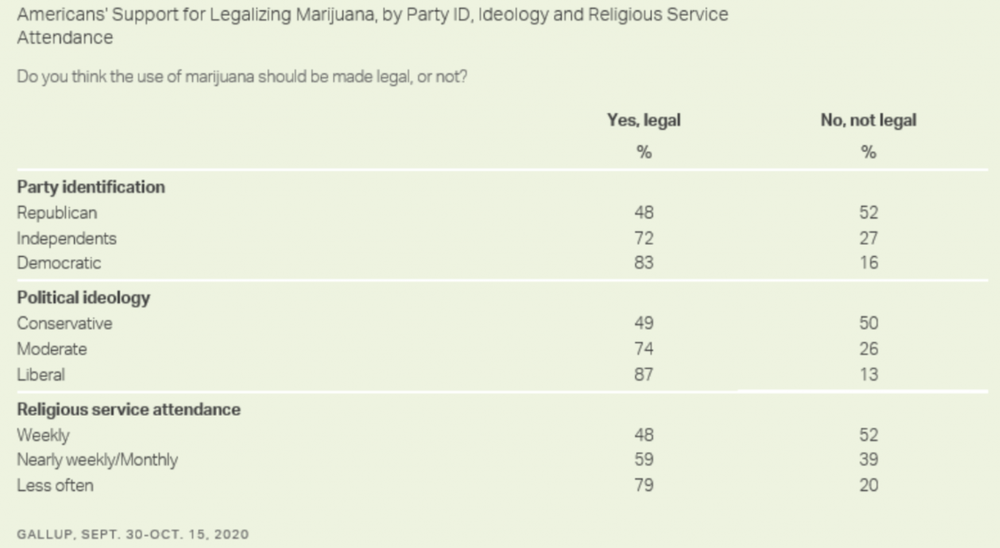
“The trajectory of the public's support for the legalization of marijuana has coincided with an increasing number of states approving it,” Gallup said. “It is not entirely clear whether the shift in public opinion has caused the change in many state laws or vice versa. Given recent trends, more states are likely to legalize recreational marijuana in the future. Considering the high level of public support for such a measure, a change in federal policy could even occur.”
Lawmakers and advocates have similarly made the case that the 2020 election results for cannabis reform will bolster federal reform efforts, regardless of the political makeup of Congress or the presidency.
“This is what voters want. They're not partisan issues, it's an opportunity for Republicans to be able to make progress in their red states and bring people together at a time of division,” Rep. Earl Blumenauer (D-OR) told Marijuana Moment on Thursday. “I think you're going to watch people understand what just happened last night, and it is a continuation of progress that's been going on since 1996. I think it's going to be much easier [to pass reform] in the new Congress, with Republicans and Democrats, both in the House and Senate.”
CarolKing
Always in search of the perfect vaporizer
https://norml.org/news/2020/11/19/c...yields-a-positive-drug-test-for-carboxy-thc/d Smoking of CBD-Rich Cannabis Yields a Positive Drug Test for Carboxy-THC
Italian investigators assessed toxicological screens in a single subject who inhaled one CBD-rich cigarette per day (six percent CBD and 0.2 percent THC) over a 26-day period.
Investigators reported that the subject tested positive for the presence of carboxy-THC in urine at levels above 15ng/ml by the fifteenth day of the trial. By contrast, repeated dosing was not associated with a positive oral fluid test for THC. Hair samples also tested negative for both the presence of THC and carboxy-THC.
Authors concluded: “[T]his study based on a single subject suggests that the repeated exposure to CBD-rich cannabis (containing small amounts of THC) can generate positive results when biological samples are tested. In particular, positive urine results for THC-COOH, using the confirmatory cut-off of 15 ng/mL, were obtained after a 15-days period of consumption. These results show the potential risk for chronic CBD-rich cannabis users of failing a urine drug test.”
The report’s findings are consistent with those of a recently published paper in JAMA Psychiatry reporting that the consumption of hemp-derived CBD products that comport with federal standards (containing no more than 0.3 percent THC) can similarly trigger a positive urine test result for marijuana exposure.
Full text of the study, “Determination of cannabinoids in urine, oral fluid, and hair samples after repeated intake of CBD-rich cannabis by smoking,” appears in Forensic science International.
Case Report: Sustained Smoking of CBD-Rich Cannabis Yields a Positive Drug Test for Carboxy-THC
- BY NORML
- POSTED ON NOVEMBER 19, 2020
Italian investigators assessed toxicological screens in a single subject who inhaled one CBD-rich cigarette per day (six percent CBD and 0.2 percent THC) over a 26-day period.
Investigators reported that the subject tested positive for the presence of carboxy-THC in urine at levels above 15ng/ml by the fifteenth day of the trial. By contrast, repeated dosing was not associated with a positive oral fluid test for THC. Hair samples also tested negative for both the presence of THC and carboxy-THC.
Authors concluded: “[T]his study based on a single subject suggests that the repeated exposure to CBD-rich cannabis (containing small amounts of THC) can generate positive results when biological samples are tested. In particular, positive urine results for THC-COOH, using the confirmatory cut-off of 15 ng/mL, were obtained after a 15-days period of consumption. These results show the potential risk for chronic CBD-rich cannabis users of failing a urine drug test.”
The report’s findings are consistent with those of a recently published paper in JAMA Psychiatry reporting that the consumption of hemp-derived CBD products that comport with federal standards (containing no more than 0.3 percent THC) can similarly trigger a positive urine test result for marijuana exposure.
Full text of the study, “Determination of cannabinoids in urine, oral fluid, and hair samples after repeated intake of CBD-rich cannabis by smoking,” appears in Forensic science International.
'Longest-serving cannabis offender' to be released early from 90-year prison sentence
Richard DeLisi was sentenced in 1989 for conspiring to traffic more than 100 pounds of marijuana into the U.S. from Jamaica.After serving more than three decades in prison for nonviolent cannabis offenses, a 71-year-old man is hoping to spend Christmas this year with his family.
Richard DeLisi, who was sentenced in 1989 to 90 years in prison for conspiring to traffic more than 100 pounds of cannabis into the U.S. from Jamaica, could be released as early as Dec. 4 amid failing health and the worsening coronavirus pandemic, according to the Florida Department of Corrections.
He is described as the "longest-serving cannabis offender in the country" by the Last Prisoner Project, a nonprofit organization working towards criminal justice reform for marijuana-related crimes. Lawyers from the Last Prisoner Project have been working pro bono to help secure DeLisi’s release from South Bay Correctional Facility in Palm Beach County, where hundreds of inmates have tested positive for the coronavirus since March.
“It has been a lifetime of heartache and loss,” said DeLisi’s son, Rick DeLisi. “We look forward to making the memories that a family should be able to make. I can’t wait to know my father is a free man.”
Rick DeLisi was just 11 years old when his dad was sentenced to spend what amounted to the rest of his life behind bars. Relatives were initially hesitant to share the news with him, instead waiting several weeks before explaining why Richard DeLisi was unable to return his son’s phone calls.
It wasn’t until Rick DeLisi was in his 30s that he came to fully understand what happened to his father.
“It’s a heavy burden,” he said. “You constantly hope for some change, and when you put in these petitions, these clemency appeals, and nothing ever budges at all, you feel like you're never going to unlock the door.”
In the time Richard DeLisi has been imprisoned, many of his loved ones have died, including his parents, his wife and one of his sons. His daughter was badly injured in a car accident and remains paralyzed.
The threat of coronavirus has spread as DeLisi’s health declined. He has diabetes, hypertension, arthritis and has suffered a series of mini strokes, putting him in the highest risk category if he were to contract Covid-19.
As of Thursday, 421 inmates and 86 staff had tested positive at South Bay Correctional Facility, according to the Florida Department of Corrections. Statewide, nearly 17,000 inmates have tested positive. Currently, no inmates are in medical isolation at the prison where DeLisi is detained.
Advocates for DeLisi have worked for decades to secure his early release from prison, citing both health and legal concerns. They have argued that he was never charged or convicted of a violent crime and was instead used as an example by an overzealous judge who wanted to send a warning to other would-be drug dealers.
But the drug DeLisi was convicted of conspiring to sell is now legal in 15 states, two territories and Washington, D.C. Florida, where DeLisi was convicted and sentenced, is one of 34 states with a medical cannabis program.
Earlier this month, voters in five states approved measures to legalize some form of cannabis use. Oregon became the first state to make possession of small amounts of harder drugs, including cocaine, heroin and methamphetamine, violations not punishable by jail time.
Despite the changing attitudes surrounding drug use, law enforcement officers made 663,000 arrests for cannabis-related offenses in 2018, which amounted to 40 percent of all drug arrests that year, according to FBI data.
As more states decriminalize and legalize cannabis, draconian prison sentences need to be revisited, said Chiara Juster, a marijuana criminal reform advocate and lawyer on DeLisi’s legal team.
“Across the nation, cannabis won wherever she was on the ballot this year,” Juster said. “That reflects the broader acknowledgment that the war on drugs has been the war on people. We have a moral obligation to decarcerate these prisoners.”
DeLisi and his older brother, Ted DeLisi, were convicted by a Polk County judge of trafficking cannabis, conspiracy to traffic cannabis and violating the Racketeer Influenced Corrupt Organization Act, a federal law passed in 1970 that allows for added criminal penalties for acts performed as part of a criminal organization.
Ted DeLisi appealed his conspiracy conviction and was released from prison in 2013. Richard DeLisi appealed that same year but was unsuccessful.
Prosecutors argued throughout the brothers’ two-week trial that Richard DeLisi was the “mastermind” of an elaborate plot to fly in 1,500 pounds of cannabis from Jamaica to the U.S. But lawyers for DeLisi said he was the victim of entrapment. A trusted friend who had become an informant for Polk County law enforcement devised the plan, not DeLisi, his lawyers argued in court.
According to court transcripts, lawyers representing the brothers expected their sentences to be between 22 and 27 years, which would have been consistent with the sentencing scoresheet adopted by Florida’s judicial system in the 1980s. Judge Dennis Maloney, however, sentenced the brothers to three consecutive 30-year sentences.
It was the same year that former President George H.W. Bush created the Office of National Drug Control Policy and appointed the nation’s first drug czar. In his first nationally televised address from the Oval Office, Bush called drugs "the gravest domestic threat facing our nation."
"Who's responsible? Let me tell you straight out — everyone who uses drugs, everyone who sells drugs, and everyone who looks the other way," he said in the 1989 address.
The war on drugs was in full swing when the DeLisis were sentenced just two months later.
“They made an example out of him,” said Mariah Daly, a legal fellow with the Last Prisoner Project. The racketeering act "was originally enacted to take care of mobsters, but it’s being used for low-level drug offenders.”
Rick DeLisi, Richard’s son, now lives in Amsterdam and has a difficult time reconciling his father’s prison sentence with changing attitudes surrounding cannabis. He said he doesn’t know many people who haven’t at least tried cannabis and calls the American prison system “a vampire that feeds off those at the bottom.”
“I can’t even count the money we’ve spent trying to get him out,” he said.
When Richard DeLisi is finally released from prison, Rick DeLisi intends to pack up his family and fly to the U.S. despite the pandemic.
“I need to receive him," he said. "I need my 1-year-old to know her grandpa.”
https://c5-glo.glomtrk5.com/t/clk?i...16Jv1EnVteTvJr2n1nXT6MeXe8j13Zbn8cpEPORyCXoD4
Marijuana Legalization Opponents Ask Courts To Overturn Voters’ Will In Several States
Unable to sway public opinion and persuade voters to reject marijuana legalization on Election Day, prohibitionists have taken a different new in their efforts to block state-level reform: litigation.
In three states, there are lawsuits pending that seek to overturn voter-approved legalization initiatives. And in one state, cannabis opponents succeeded this year in preventing voters from even having a chance to decide on a reform measure.
While every single drug policy reform initiative that made the ballot passed in red and blue states alike this month, prohibitionists increasingly seem to be giving up the public messaging fight to change voters’ minds and are instead resorting to the courts, challenging reform measures on largely technical matters.
Those legal fights are ongoing in Mississippi, Montana and South Dakota, all of which ultimately legalized cannabis is some form on Election Day.
The Mississippi Supreme Court recently set deadlines for legal filings in a case from the city of Madison challenging the medical cannabis initiative that overwhelmingly passed with 73 percent of the vote. The suit was filed days before the election, with the mayor not weighing in on the merits of the measure but contending that its placement on the ballot was unconstitutional due to statutory signature gathering requirements.
The secretary of state and attorney general condemned the action as a “woefully untimely” lawsuit. The Supreme Court said the filers have until December 7 to submit written arguments and the secretary of state has until December 28 to respond.
Over in Montana, opponents of a voter-approved initiative to legalize cannabis for adult usetried to get the state Supreme Court to invalidate the proposal ahead of the vote, but the justices rejected that request, arguing that they failed to establish the urgency needed to skip the lower court adjudication process. They didn’t rule on the merits, however.
The plaintiffs are now pursuing action in a lower court, arguing that the statutory proposal unlawfully appropriates funds, violating a portion of the state Constitution that prohibits such allocations from being included in a citizen initiative.
In South Dakota, Pennington County Sheriff Kevin Thom and state Highway Patrol Superintendent Col. Rick Miller filed a lawsuit in the state’s Sixth Judicial Circuit Court last week, claiming that the proposal to legalize marijuana that passed with 54 percent of the vote should be invalidated. The suit, which is partly paid for with state funds, says the constitutional amendment violates a 2018 requirement that “no proposed amendment may embrace more than one subject.”
In September, reform opponents successfully bumped an initiative to legalize medical cannabis off of Nebraska’s ballot on what essentially amounts to a technicality.
While the campaign collected enough signatures to qualify the measure, the state Supreme Court shut it down following a legal challenge. It determined that the measure violated the state’s single-subject rule, much to the disappointment of advocates. But activists have already started petitioning to get a simplified version of proposal on the state’s 2022 ballot.
Arizona activists, who succeeded in getting a legalization measure approved on Election Day, had a different experience following a legal challenge in the summer. Opponents there filed suit arguing that the 100-word summary of the initiative misled voters, but that argument did not hold up in court.
Legalization opponents point out that with voter support for marijuana reform increasing, prohibitionists are now left with few options to stop popular reforms.
NORML Deputy Director Paul Armentano said in a blog post that the opposition lawsuits are “cynical, and arguably frivolous, attempts to undermine the democratic process.”
“Legalization opponents have shown time and time again that they cannot succeed in either the court of public opinion or at the ballot box,” he said. “Thus, they are now asking judges to set aside the votes of over a million Americans in a desperate effort to override undisputed election outcomes. Whether or not one supports marijuana legalization, Americans should be outraged at these overtly undemocratic tactics.”
Marijuana Bill Up For House Vote Could ‘Reverse’ Federal-State Policy Gap, Congressional Research Service Says
A bill to federally legalize marijuana that is scheduled for a House vote next week could “reverse” the current cannabis policy gap that exists between states and the federal government, a new Congressional Research Service (CRS) report says.
In an analysis of the Marijuana Opportunity, Reinvestment and Expungement (MORE) Act that was published on Wednesday, CRS described the various complications resulting from ongoing federal prohibition as more states opt to legalize cannabis for medical or recreational purposes. The research agency said the legislation could inadvertently create a new schism where federal policy would be more progressive than those of certain states.
That’s because the bill does not require states to stop criminalizing cannabis, and so jurisdictions with prohibition still on the books could continue to punish people over marijuana even as such activity is legalized at the federal level.
“If the MORE Act became law, it could create a new divide between federal and state law—essentially the reverse of the current marijuana policy gap, since federal marijuana law would become less strict than some state laws,” CRS wrote. “The MORE Act could also highlight the inconsistency between marijuana laws in different U.S. jurisdictions by repealing the uniform federal prohibition and leaving in place a patchwork of varying state laws.”
The MORE Act, whose lead sponsor is Judiciary Committee Chairman Jerrold Nadler (D-NY), would federally deschedule cannabis, expunge the records of those with prior marijuana convictions and impose a federal five percent tax on sales, revenue from which would be reinvested in communities most impacted by the drug war.
The legislation would also create a pathway for resentencing for those incarcerated for marijuana offenses, as well as protect immigrants from being denied citizenship over cannabis and prevent federal agencies from denying public benefits or security clearances due to its use.
“Congress may be content to allow states to experiment with varying approaches to marijuana regulation,” CRS said. “In the alternative, Congress might prefer a more uniform approach, whether that approach is to criminalize or decriminalize marijuana, or something in between. However, while Congress can pass legislation creating a uniform federal policy, there are limits to its ability to affect state law.”
The agency said that while lawmakers lack the “constitutional authority to alter state criminal law,” they could “preempt state law through Commerce Clause legislation” or “encourage states to change their laws through the use of the spending power.”
To that end, while the MORE Act does not create a federal regulatory structure for cannabis or force states to change their own laws, it does include provisions that incentivize the adoption certain local reform policies. For example, it offers federal funding for “eligible states” that take steps to expunge prior cannabis convictions and stop penalizing people on parole for marijuana-related offenses.
“Congress could also invoke its spending power to encourage states to regulate marijuana more stringently, and has previously used the spending power to shape drug policy in targeted ways,” CRS said.
These and other considerations will likely be the subject of significant debate when the House takes up the MORE Act next week.
House Majority Leader Steny Hoyer (D-MD) announced on Friday that the bill will be taken on the floor as soon as Wednesday.
He had previously said this summer that the chamber would vote on the legislation in September, but that plan was postponed following pushback from certain centrist Democrats who worried about the optics of advancing cannabis reform before passing another coronavirus relief package.
The bill cleared Nadler’s more than a year ago and has been awaiting floor action since.
Even if the far-reaching reform does pass in the Democratic-controlled chamber, as it’s expected to with some bipartisan support, it remains unlikely that the Senate will follow suit, at least during this Congress. Majority Leader Mitch McConnell (R-KY) is a champion of the hemp industry but staunchly opposes further marijuana reform.
Even so, a symbolic vote for legalization could send a strong signal to the incoming Biden administration. Vice President-elect Kamala Harris (D-CA) is the lead Senate sponsor of the MORE Act, but she’s indicated she will not necessarily proactively push the former vice president to evolve further on cannabis reform.
Given Biden’s former approach to championing punitive anti-drug legislation as a senator and his ongoing obstinance on marijuana legalization at a time when polls show that a clear majority of Americans favor the policy change, there remains some skepticism about his willingness to make good on his campaign promises to achieve more modest reforms he has endorsed, such as decriminalizing possession and expunging records.
A transition document the incoming Biden-Harris administration released this month left out mention of those cannabis pledges.
That said, the president-elect has conceded that his work on punitive anti-drug legislation during his time in Congress was a “mistake.”
For what it’s worth, Rep. Earl Blumenauer (D-OR) told Marijuana Moment in August that “the Biden administration and a Biden Department of Justice would be a constructive player” in advancing legalization.
CRS, in its new report, also discussed broader drug policy reform efforts taking place at the state level and locally, such as Oregon’s recent vote to decriminalize possession of all currently illicit drugs. The agency noted moves to decriminalize psychedelics specifically in Washington, D.C., too.
These “current trends suggest that there may be a broader movement toward decriminalizing controlled substances,” CRS said. “Comprehensively addressing such changes is outside the scope of the MORE Act, but Congress may wish to monitor developments in this area when considering future legislation.”
These States Could Have Marijuana Legalization On Their 2022 Ballots
Five states legalized marijuana in some form on Election Day this year. When those newly approved laws take effect, about one-third of the nation’s population will live in a state where cannabis is legal for medical or recreational purposes. Now, advocates are already hard at work formulating plans and collecting signatures to extend that success to even more states during the 2022 midterms.
For background, every single marijuana measure placed on state ballots this year passed: Arizona (recreational), Mississippi (medical) Montana (recreational), New Jersey (recreational), South Dakota (recreational and medical). The victories represent a continuation of the state-level reform movement that has consistently expanded in election after election.
But while advocates see this momentum as building pressure for federal policy change, they aren’t giving up statewide pushes.
Nebraska and Idaho activists are already in the process of qualifying medical marijuana measures for midterm ballots in 2022, for example. And it’s unlikely that those will be the only states were voters will get to decide on cannabis reform in two years.
Matthew Schweich, the deputy director of the Marijuana Policy Project who was involved in several of the successful reform initiatives this year, told Marijuana Moment that the election results make him optimistic that “there’s a path to victory in multiple states [in 2022] and we just need to survey the landscape.”
“The biggest takeaway is that we can probably run competitive campaigns on either issue [medical or recreational] in any state,” he said. “It’s just a question of what are the strongest opportunities to make the greatest positive impact given the resources available.”
Here’s a rundown of the most likely states where marijuana legalization will make the ballot two years from now.
Recreational:
Arkansas
Activists attempted to put a legalization measure on the 2020 ballot—and even secured a procedural victory when a court mandated that the state accept signatures that were not collected in-person or notarized due to the coronavirus pandemic. But they were nonetheless unable to garner enough petitions to qualify to put the issue before voters this year.Although Arkansas voters already approved medical cannabis in 2016, the effort to put up another fight for broader legalization could be bolstered by voters in neighboring Mississippi, another traditionally conservative state, approving a medical marijuana initiative on Election Day this year, which signaled that reform has momentum regionally.
Florida
Florida activists announced back in January that, due to restrictive ballot qualification policies in the state, they will be pursuing a marijuana legalization initiative in 2022, rather than this year as initially planned.The Make It Legal Florida campaign filed a lawsuit with the state’s Supreme Court at the beginning of the year, alleging that a new law that imposes restrictions on the signature gathering process is unconstitutional. They didn’t prevail in that case, however, and the legalization measure didn’t make the ballot.
Now, advocates will turn their attention to 2022, though it is worth noting that cannabis reform measures have traditionally had better success in presidential election years rather than during midterms—and Florida rules require an initiative to get 60 percent of the vote in order to pass rather than a simple majority. While a Florida medical cannabis measure fell just short of the supermajority needed for approval during the 2014 midterms, a follow-up attempt two years later was successful.
Missouri
There was a push to put recreational cannabis legalization on the state’s 2020 ballot, but activists announced in March that the coronavirus pandemic meant they would have to suspend their campaign. While they have not made any official decisions, a spokesperson said that “it is likely we will return to pursue this goal in 2022.”Missouri voters approved a ballot measure to legalize medical marijuana in 2018, and dispensaries made the state’s first cannabis sales to patients in October.
North Dakota
A campaign to legalize cannabis in North Dakota worked hard to put the reform measure on this year’s ballot. They ceased campaign activities amid the COVID-19 outbreak in April, but considered starting back up in a limited capacity when businesses started reopening. Ultimately, however, advocates stopped pushing for the proposal and conceded that it would more likely appear on the 2022 ballot.“Our whole thing is about ending people going to prison, our whole point is about ending the dangers of the war on drugs, so it would be irresponsible for us to endanger people while we try to do it,” a spokesperson said in May. “Two wrongs don’t make a right. We’re going to try, but [qualifying for November] likely still isn’t in the cards. That just means we have more time to get prepared to push for that 2022 date.”
Meanwhile, advocates are stepping up the push to get lawmakers to enact marijuana reform bills following a formal review of the issue conducted by the legislature’s Judiciary Committee.
Ohio
Ohio was another example of a state where activists attempted and failed to qualify an initiative to legalize marijuana for adult use, due in large part to restrictions resulting from the COVID-19 outbreak.A proposed legal cannabis measure was filed in March, just before social distancing measures that made signature collection all but impossible were enacted. Now, advocates are looking ahead to 2022, when it may be possible to mount a successful ballot qualification bid.
Meanwhile, a group called the Sensible Movement Coalition has been pursing local marijuana decriminalization initiatives through municipal ballots. They tried to go through the courts to get the state to allow digital signature gathering amid the pandemic—and at first they got a victory when a federal judge ruled in their favor—but an appeals court shut that lawsuit down in June. That said, they did succeed in getting four more Ohio cities to adopt local measures to decriminalize cannabis during the November election.
Oklahoma
Oklahoma activists filed a proposed ballot measure to legalize cannabis for adult use in December. But they ultimately withdrew that petition in August—again, because of the coronavirus pandemic.“We have been put in a position of choosing between attempting to give Oklahomans an opportunity to adopt critical marijuana and criminal justice law reforms or protecting the health of ourselves and our fellow Oklahomans,” a campaign spokesperson said. “As necessary as these reforms are for Oklahoma, we cannot in good conscience embark on a campaign that would require hundreds of thousands of interactions in the midst of a global pandemic.”
The campaign said they soonest they would take up the reform proposal would be in 2022. Voters in the state approved a medical marijuana ballot measure during a 2018 primary election. This year, a bill to significantly expand the state’s medical cannabis program advanced through the legislature, but the governor vetoed it and lawmakers declined to pursue an override before adjourning for the session in May.
Medical:
Idaho
In Idaho, advocates behind a measure to legalize medical marijuana tried to make the 2020 ballot, but they gave up that effort due to COVID-related signature gathering complications and the state’s refusal to provide petitioning accommodations.Activists have already filed a petition to put the issue before voters in 2022, however.
“We’re really excited about the 2022 campaign because what we’ve proven in 2020 is that the people of Idaho are very much ready for medical marijuana to be passed,” campaign spokesperson and longtime reform advocate Russ Belville told Marijuana Moment last month. “We know the signatures are out there, we know that people are ready for this.”
Nebraska
Nebraska activists have already started petitioning to get a medical cannabis measure on the state’s 2022 ballot.While the campaign collected enough signatures to qualify a reform initiative for this year, the state Supreme Court shut it down following a legal challenge. The justices determined that the measure violated the state’s single-subject rule, much to the disappointment of advocates.
To avoid getting blocked under the single-subject rule, the language of the new proposal concisely states: “Persons in the State of Nebraska shall have the right to cannabis in all its forms for medical purposes.” It has no regulations.
“Though we profoundly disagree with the Nebraska Supreme Court’s decision in September to remove our previous initiative from the ballot, we took good notes,” the campaign said in October. “They argued the previous language violated the single subject rule, so now we’ve drafted one, simple sentence to legalize medical cannabis.”
2022 already on my mind. #MedicalMarijuana#Marijuana pic.twitter.com/XDR7jNCnVM
— Senator Adam Morfeld (@Adam_Morfeld) November 5, 2020
State Sen. Adam Morfeld (D), a chief petitioner for the initiative, told Marijuana Moment that the campaign “carefully crafted our new language to be in compliance with the new precedent set forth by the Supreme Court in September so we are confident it complies with the single subject rule.”
“Medical marijuana is incredibly popular across partisan and ideological lines in Nebraska and we are confident once we have the signatures and it is on the ballot that it will pass by a wide margin,” he said.
Would have, could have, should have. We were pursuing Medical Marijuana because of the compelling need for it by our constituents and because of the broad political support. Would have, could have, should have. We will continue to fight and a lot can happen in two years — Senator Adam Morfeld (@Adam_Morfeld) November 6, 2020
There’s also some speculation that a proposal to legalize for adult use could be in the cards—but that’s less likely.
Beyond The Ballot
Aside from voter-initiated ballot measures, there are multiple states where top lawmakers have said they’re serious about pursing cannabis legalization bills in 2021, including New Mexico, New York and Rhode Island. In Connecticut, the incoming House speaker said that if there aren’t enough votes in the legislature to pass a legalization bill next year, lawmakers will put it on the ballot for voters to decide as a referendum.United Nations To Vote On Marijuana Rescheduling And CBD Issues This Week, With U.S. Backing Some Reforms
A key United Nations (UN) commission will vote on a series of World Health Organization (WHO) recommendations concerning international marijuana reform this week. And the U.S. is in favor of the boldest policy change.
UN’s Commission on Narcotic Drugs (CND) has held numerous meetings on the proposals—including removing cannabis from the most restrictive global drug scheduling category under a global treaty—since WHO made its six recommendations last year. Now, after several delays, CND is finally scheduled to meet to decide on the measures on Wednesday.
Advocates are generally encouraged by the development, arguing that a vote in favor of the reforms will promote research into the therapeutic potential of cannabis. However, they say removing marijuana from its current international Schedule IV status does not go far enough and means that many member nations will continue to criminalize the plant.
Here are each of WHO’s cannabis recommendations:
1. Remove marijuana from Schedule IV of the 1961 Single Convention.
2. Add THC and dronabinol (synthetic THC medication) to Schedule I of the 1961 Convention and, if approved, delete them from Schedule II of the 1971 Convention.
3. If the second recommendation is adopted, add tetrahydrocannabinol to Schedule I of the 1961 Convention and, if approved, delete it from Schedule I of the 1971 Convention.
4. Delete “extracts and tinctures of cannabis” from Schedule I of the 1961 Convention.
5. Add footnote to clarify that CBD products containing no more than 0.2 percent THC are not subject to international control.
6. Add “preparations containing dronabinol” to Schedule III of the 1961 Convention.
Last month, the U.S. government said it is backing the WHO recommendation to remove marijuana from the most restrictive global drug scheduling category—though it’s opposing separate cannabis reform proposals, including the one to clarify that CBD is not under international control.
John Walsh, director of drug policy for Washington Office on Latin America (WOLA), told Marijuana Moment that this upcoming vote is “momentous,” especially as “this is the first time that the UN scientific bodies has assessed placing cannabis and drug control schedules.”
‘And it’s extremely significant that the United States is supporting a recommendation to remove cannabis from Schedule IV, which strongly discourages medical uses of cannabis, even if it doesn’t outright prohibit it,” he said.
Of principal concern to advocates is that while marijuana would be removed from Schedule IV under the 1961 Single Convention—the most strict international category—it would maintain its status as a Schedule I controlled substance if the panel accepts the recommendation. (The international scheduling system differs from that of the U.S. in that the country’s most restrictive category is Schedule I.)
But despite supporting that recommendation, the U.S. circulated a proposed joint statement to other member states that claims consensus on the notion “that cannabis is properly subject to the full scope of international controls of the 1961 Single Convention, due in particular to the high rates of public health problems arising from cannabis use and the global extent of such problems, as identified in the critical review by WHO.”
It also stipulates that “no Party shall be precluded from adopting measures of control more strict or severe than those required as a result of this decision, if such measures in its opinion are necessary or desirable for the protection of the public health or welfare.” The language seems to attempt to leave room for countries to continue enforcing more restrictive cannabis policies regardless of international rules.
In an email obtained by Marijuana Moment, a State Department official said that the U.S. “believes, to demonstrate unity, every CND member and observer could ideally join the statement below, regardless of how their government will vote.” They also plan to proceed with filing the statement even if no other member states join them.
The statement represents a “disconnect” from the country’s planned vote in favor of removing marijuana from the international body’s most restrictive drug classification, Walsh said.
“Civil society had called for, and welcomed, this long overdue review process—but many have been critical of some of the recommendations,” drug policy reform advocates said in a media advisory. “While recommendations on medical cannabis and CBD are certainly positive steps, profound concerns have been raised around leaving cannabis in Schedule I of the 1961 Convention.”
“This recommendation is at odds with The Who Expert Committee on Drug Dependence’s clear finding that cannabis was less harmful than other drugs included in that schedule (heroin and cocaine),” the advisory, prepared by advocacy groups Transform Drug Policy Foundation, Transnational Institute, International Drug Policy Consortium and WOLA, said.
“Regardless of the outcome of the votes on 2 December, this historic review process has demonstrably failed to implement a much-needed modernization of an outdated and malfunctioning system, and to resolve key scientific, political, institutional and human rights challenges related to cannabis and its status in the international drugs control system,” they said.
Numerous health and drug policy reform groups have advocated for the more modest changes WHO proposed.
A coalition of drug policy groups told member nations in a sign-on statement that patients worldwide are “counting on you to seize the opportunity offered by WHO to update the treaties, doing all you can to ensure access to all useful medicines. Including cannabis medicines.”
“Adopting WHO’s recommendations would lead to better medications being developed and more tools for doctors to alleviate suffering while simultaneously reinforcing the UN’s relevance,” they said.
The Multidisciplinary Association for Psychedelic Studies also weighed in in favor of the recommendations.
While the WHO’s CBD recommendation would simply offer clarification that cannabidiol products containing no more than 0.2 percent THC isn’t a controlled substance under international treaties, the U.S. came down against that and several other cannabis-related proposals.
It should be noted that none of WHO’s recommendations would promote the legalization of cannabis in any country, but advocates nonetheless seem that as a step forward from the status quo.
“This is super, super meaningful. But I don’t want to overstate it,” Michael Krawitz, a U.S. Air Force veteran and legalization advocate who has spent years working to reform international drug treaties, told Marijuana Moment. “I’ve been cautioning really hard to member states to not fall into this trap that the opposition fell into on [on California’s 1996 medical cannabis initiative] of overstating what this does in an effort to try to stop it—and then vicariously creating expectations in people’s minds that this actually does much more than it does.”
But the U.S.’s expected support for the proposal to remove marijuana from Schedule IV represents a departure from its position as articulated in a government document that Marijuana Moment obtained earlier this year. The document stated that it’s “possible that civil society, the media, and the general public will view deleting cannabis from Schedule IV as a first step toward widespread legalization of marijuana use, especially without proper messaging.”
Meanwhile, if the recommendation on CBD is adopted, it could potentially have far-reaching implications in the U.S. In 2018, the FDA determined that CBD does not meet the criteria for federal control—except for the fact that international treaties to which the U.S. is party could potentially be construed as requiring it.
The U.S. does intend to back the fourth WHO recommendation on deleting cannabis extracts and tinctures from Schedule I of the 1961 Convention, according to advocates familiar with the delegation’s thinking.
FDA has on several occasions solicited public input to shape the government’s position on the international scheduling of marijuana and cannabinoids. The agency initially requested feedback on the proposal in March 2019 and then reopened that comment period five months later.
House Leaders Propose Changes To Federal Marijuana Legalization Bill Up For Floor Vote This Week
A key House committee has scheduled a Wednesday hearing to advance a bill to federally legalize marijuana toward a full floor vote, which could then happen as soon as Thursday. Meanwhile, leaders in the chamber are proposing an amendment that would make several changes to the cannabis legislation.
Among the most significant revisions would be to the tax-related provisions of the bill.
The Rules Committee’s move to take up the Marijuana Opportunity, Reinvestment and Expungement (MORE) Act follows Majority Leader Steny Hoyer (D-MD) announcement that the chamber would be holding a floor vote on the bill before the end of the year.
Judiciary Committee Chairman Jerrold Nadler (D-NY), the lead sponsor of the bill, transmitted it to Rules with the series of modifications—many of them technical in nature. But beyond the tax changes, the newly proposed language also reaffirms the regulatory authority of certain federal agencies such as the Food and Drug Administration (FDA) and clarifies that cannabis can still be included in drug testing programs for federal workers.
Other members of the House are likely to file proposed amendments as well, though the Democratic majority of the Rules panel will determine which ones can be made in order for floor votes later this week.
As originally drafted, the legislation would have imposed a five percent tax on marijuana products, revenue from which would be used in part to fund a grant program to support communities disproportionately impacted by the war on drugs. In Nadler’s amendment, that language is being removed and replaced with text that more closely reflects a separate descheduling bill, the Marijuana Revenue and Regulation Act.
The modified tax provisions of the MORE Act would make it so cannabis would be federally taxed at five percent for the first two years after implementation and then increased by one percent each year until reaching eight percent. After five years, taxes would be applied to marijuana products based on weight rather than price.
At its core, the MORE Act would federally deschedule cannabis from the Controlled Substances Act and expunge the records of those with prior marijuana convictions. The descheduling provisions would be retroactive.
The bill would also create a pathway for resentencing for those incarcerated for marijuana offenses, as well as protect immigrants from being denied citizenship over cannabis and prevent federal agencies from denying public benefits or security clearances due to its use.
A new Cannabis Justice Office under the Justice Department would be responsible for distributing funds providing loans for small cannabis businesses owned and controlled by socially and economically disadvantaged individuals. The bill also seeks to minimize barriers to licensing and employment in the legal industry.
While the bill still calls for the establishment of a Community Reinvestment Grant Program, the new leadership amendment would remove a line calling for it to specifically fund “services to address any collateral consequences that individuals or communities face as a result of the War on Drugs.”
Tax dollars appropriated to that program would instead more generally go to job training, legal aid for criminal and civil cases such as those concerning marijuana-related expungements, literacy programs and youth recreation and mentoring services, among other programs.
The definition of people impacted by the drug war who could be eligible for aid is also being changed to narrow the scope. At first it included those who have “been arrested for or convicted of the sale, possession, use, manufacture, or cultivation of cannabis or a controlled substance,” but now it only extends to marijuana and not other illicit drugs.
Other changes included in Nadler’s latest revision include one requiring FDA and the U.S. Department of Health and Human Services (HHS) to hold public meetings on “regulation, safety, manufacturing, product quality, marketing, labeling, and sale of products containing cannabis or cannabis-derived compounds” within one year of the bill’s enactment.
The language is also being updated to reflect the current number of states where marijuana is legal for medical or recreational purposes, clarify that FDA and HHS maintain their authorities to regulate cannabis products and stipulate that federal agencies can continue to include cannabis in employee drug testing. A conforming amendment would clarify that the U.S. Department of Transportation could continue to require drug testing for workers in safety sensitive positions.
The revised version also stipulates that funding can be made available to “connect patients with substance use disorder services” and apply to “individuals who have been arrested for or convicted of the sale, possession, use, manufacture, or cultivation of a controlled substance other than cannabis (except for a conviction involving distribution to a minor).”
The proposal also deletes from the definition of substance misuse treatment language stating that it would be an “evidence-based, professionally directed, deliberate, and planned regimen including evaluation, observation, medical monitoring, harm reduction, and rehabilitative services and interventions such as pharmacotherapy, mental health services, and individual and group counseling, on an inpatient or outpatient basis, to help patients with substance use disorder reach remission and maintain recovery.”
There are also a number of technical and conforming changes in the proposal, as well as the removal of the word “most” from “individuals most adversely impacted by the War on Drugs” when it comes to determining eligibility for the new programs and services created by the legislation.
In a new report on the bill that was submitted by the Democratic majority in Judiciary, members said cannabis enforcement “has been a key driver of mass criminalization in the United States” and the “drug war has produced profoundly unequal outcomes across racial groups, manifested through significant racial disparities throughout the criminal justice system.”
“The higher arrest and incarceration rates for communities of color do not reflect a greater prevalence of drug use, but rather the focus on law enforcement on urban areas, lower income communities, and communities of color,” they wrote.
Further, the “collateral consequences of even an arrest for marijuana possession can be devastating, especially if a felony conviction results.”
“Those arrested can be saddled with a criminal conviction that can make it difficult or impossible to vote, obtain educational loans, get a job, maintain a professional license, secure housing, secure government assistance, or even adopt a child,” the report states. “These exclusions create an often-permanent second-class status for millions of Americans. Like drug war enforcement itself, these consequences fall disproportionately on people of color. For non-citizens, a conviction can trigger deportation, sometimes with almost no possibility of discretionary relief.”
Rep. Jim Jordan (R-OH), GOP ranking member on the panel, wrote the minority opinion in the report.
He argued that the MORE Act “disregards established science” and “would open the floodgates to marijuana cultivation, distribution, and sale within the United States—allowing bad actors and transnational criminal organizations to further exploit America’s addiction crisis.”
The congressman complained that the legislation—which he called “an extreme and unwise measure”—wouldn’t impose limits on THC concentration or ban flavored cannabis products, and he said it “fails to funnel any tax revenue towards a public awareness campaign to discourage teen use of marijuana, modeled on successful anti-tobacco campaigns.”
He also claimed it “does nothing to help the Federal government and scientific community to understand the effects of marijuana usage.”
Vice President-elect Kamala Harris (D-CA) is the lead sponsor of the Senate companion version of the MORE Act.
One provision of the bill requires that any uses of the words “marijuana” or “marihuana” in U.S. Code or regulations be replaced with the term “cannabis”—despite the fact that the legislation has “marijuana” in its own title.
The Congressional Research Service released an analysis of the MORE Act last week, finding that the bill’s passage could “reverse” the current cannabis policy gap that exists between states and the federal government.
That’s because the bill does not require states to stop criminalizing cannabis, and so jurisdictions with prohibition still on the books could continue to punish people over marijuana even as such activity is legalized at the federal level.
Even if the legislation does pass in the Democratic-controlled chamber, as it’s expected to with some bipartisan support, it remains unlikely that the Senate will follow suit. Majority Leader Mitch McConnell (R-KY) is a champion of the hemp industry but staunchly opposes further marijuana reform.
That said, a symbolic vote for legalization could send a strong signal to the incoming Biden administration.
Given Biden’s former approach to championing punitive anti-drug legislation as a senator and his ongoing obstinance on marijuana legalization at a time when polls show that a clear majority of Americans favor the policy change, there remains some skepticism about his willingness to make good on his campaign promises to achieve more modest reforms he has endorsed, such as decriminalizing possession and expunging records.
A transition document the incoming Biden-Harris administration released this month left out mention of those cannabis pledges.
That said, the president-elect has conceded that his work on punitive anti-drug legislation during his time in Congress was a “mistake.”
For his part, Rep. Earl Blumenauer (D-OR) told Marijuana Moment in August that “the Biden administration and a Biden Department of Justice would be a constructive player” in advancing legalization.
Bcbud
Cannabis enthusiast

The US Is Sandwiched Between Two Countries With Legal Weed
The Fresh Toast - Marijuana is legal nationwide in Canada, and it could soon be in Mexico, as well. - Marijuana Legislation
Baron23
Well-Known Member
So, do you think that this might perhaps include impoverished communities in say....Appalachia? Hmmm?A new Cannabis Justice Office under the Justice Department would be responsible for distributing funds providing loans for small cannabis businesses owned and controlled by socially and economically disadvantaged individuals.
United Nations Removes Marijuana From Most Strict Global Drug Category, With U.S. Support
A key United Nations (UN) panel approved a World Health Organization (WHO) recommendation to remove marijuana from the most restrictive global scheduling category on Wednesday.
With the backing of the U.S. government, the UN’s Commission on Narcotic Drugs (CND) adopted the proposal to delete cannabis from Schedule IV of the 1961 Single Convention.
This doesn’t mean that member nations are cleared to legalize marijuana, however, as it remains under the separate Schedule I of the international drug control system. But advocates say it demonstrates an evolution in how the international community views cannabis policy, and it could promote research into the plant’s therapeutic potential.
WHO made six recommendations on global cannabis policy last year—and CND has held numerous meetings on the proposals. After numerous meetings and delays, members finally held votes at a meeting this week.
The proposal to remove marijuana from Schedule IV was arguably the most consequential of the reforms, and is the only one that was approved. (The international scheduling system differs from that of the U.S. in that the country’s most restrictive category is Schedule I.)
Here’s a rundown of the votes on the WHO’s cannabis recommendations:
APPROVED: Remove marijuana from Schedule IV of the 1961 Single Convention.
REJECTED: Add THC and dronabinol (synthetic THC medication) to Schedule I of the 1961 Convention and, if approved, delete them from Schedule II of the 1971 Convention.
REJECTED: If the second recommendation is adopted, add tetrahydrocannabinol to Schedule I of the 1961 Convention and, if approved, delete it from Schedule I of the 1971 Convention.
REJECTED: Delete “extracts and tinctures of cannabis” from Schedule I of the 1961 Convention.
REJECTED: Add footnote to clarify that CBD products containing no more than 0.2 percent THC are not subject to international control.
REJECTED: Add “preparations containing dronabinol” to Schedule III of the 1961 Convention.
The vote to move cannabis out of its restrictive scheduling status was close, with 27 countries in favor, 25 against and one abstention.
Russia, China and Pakistan were among those nations that opposed the reform.
Russia voted against all @WHO/ECDD recommendations on cannabis and cannabis-related substances. Half of the CND membership shares the same position. pic.twitter.com/Ep8m1gfnxt
— Russian Mission Vienna (@mission_rf) December 2, 2020
The U.S. said in October that it would be supporting the WHO recommendation to remove cannabis from the restrictive global drug scheduling category—though it was opposing the other cannabis reform proposals, including the one to clarify that CBD is not under international control.
Despite supporting the main scheduling recommendation, however, the country circulated a proposed joint statement to other member states that claimed consensus on the notion “that cannabis is properly subject to the full scope of international controls of the 1961 Single Convention, due in particular to the high rates of public health problems arising from cannabis use and the global extent of such problems, as identified in the critical review by WHO.”
It also stipulates that “no Party shall be precluded from adopting measures of control more strict or severe than those required as a result of this decision, if such measures in its opinion are necessary or desirable for the protection of the public health or welfare.” The language seems to attempt to leave room for countries to continue enforcing more restrictive cannabis policies regardless of international rules.
While the WHO’s CBD recommendation would simply offer clarification that cannabidiol products containing no more than 0.2 percent THC isn’t a controlled substance under international treaties, the U.S. and the vast majority of other countries voted against the measure.
The U.S.’s support for the recommendation to remove marijuana from Schedule IV represents a departure from its position as articulated in a government document that Marijuana Moment obtained earlier this year. The document stated that it’s “possible that civil society, the media, and the general public will view deleting cannabis from Schedule IV as a first step toward widespread legalization of marijuana use, especially without proper messaging.”
If the recommendation on CBD had been adopted, it could have had far-reaching implications in the U.S. In 2018, the FDA determined that CBD does not meet the criteria for federal control—except for the fact that international treaties to which the U.S. is party could potentially be construed as requiring it.
FDA has on several occasions solicited public input to shape the government’s position on the international scheduling of marijuana and cannabinoids. The agency initially requested feedback on the proposal in March 2019 and then reopened that comment period five months later.
Baron23
Well-Known Member
And the absolute only people who give a shit about this is the DEA and drug warrior politicians. I mean, we stiff the UN all of the time.....but on this we listen to them. hahaha...nay, nay I think.This doesn’t mean that member nations are cleared to legalize marijuana, however, as it remains under the separate Schedule I of the international drug control system.
And now this is funny....Pakistan, home of the greatest traditional hashish industry.....which their government definitely does NOT shut down.Russia, China and Pakistan were among those nations that opposed the reform.
|
Sponsored by |
|---|
|
|
|