THC & CBD - Promiscuous Partners With Many Receptors - Part III
Did you think that THC and CBD could only interact with just the CB1 and CB2 receptors? Think again!
Both tetrahydrocannabinol (THC) and cannabidiol (CBD) are highly promiscuous. Yes, this is an actual term in pharmacology! It means that they hit multiple pharmacologic targets within the cell instead of just one. There are many different targets, including cell surface receptors, nuclear receptors, uptake transporters, and cannabinoid binding proteins.
How common are promiscuous drugs? You see them less and less. Pharmaceutical drugs these days are optimized to have very high potency at one intended target. Molecules are chosen for their
selectivity – the ability to hit just one target without touching others. However, many older drugs (especially psychiatric ones like antidepressants and antipsychotics) do bind multiple targets. This can both contribute to their efficacy and their side effects.
Potency is an important concept that cannot be overlooked. Contrary to how it is informally used, it does
not mean how strong the effect of a drug is. Rather, it refers to what
concentration or
dose the effect is achieved at. Potency can be expressed as an
EC50 – the concentration that produces half the maximal effect, or an
IC50 – the concentration that produces half the maximal inhibition of an effect.
THC and CBD have different potencies at different targets. The importance of this is that
the higher the dose, the more targets THC and CBD will engage.
So for example, if you smoke a bit of low-CBD cannabis, the CBD levels in your body will be too low to engage many of the targets (or maybe none at all). If you take a very high dose of purified CBD oil, levels in your body could be high enough to engage many of them. The potency of other targets may be so low that engagement can only be shown in the lab and has no real significance. In a follow-up article, I will estimate what doses are needed to engage different targets.
Below, are the targets of THC and CBD that have been discovered (so far). I won’t cite every individual study, but here is a recent
review on the topic.
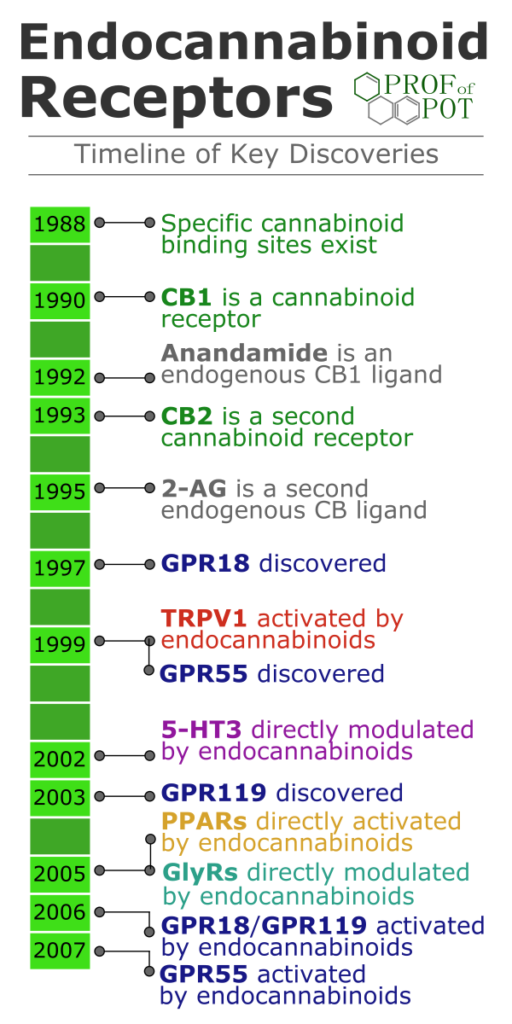
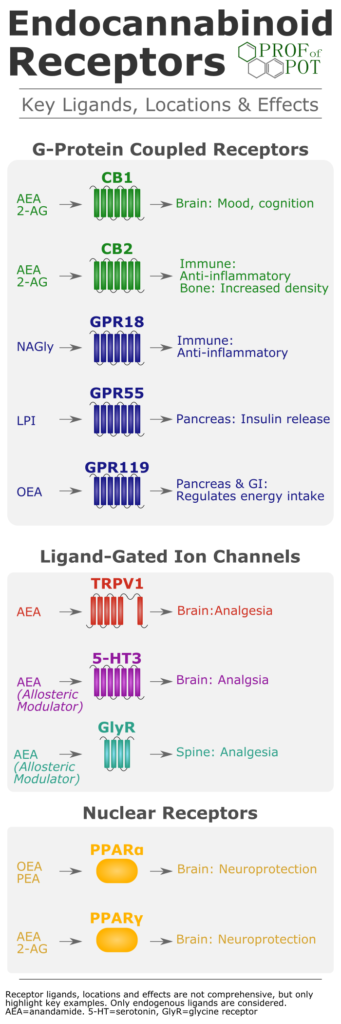
Endocannabinoid System
The
cannabinoid CB1 receptor is the most important for psychoactive effects, but has a role in a number of therapeutic effects as well, particularly
pain. THC is a potent partial agonist of CB1. However, the effect of CBD has been more difficult to determine. Originally CBD was considered to be a low-potency antagonist at CB1. However, in 2015, new results showed that CBD can also bind to an allosteric site on the CB1 receptor. At this site, CBD acts as a high potency (IC50=304 nM) negative allosteric modulator, which could reduce both the efficacy and potency of CB1 activation by THC.
Cannabinoid CB2 receptor activation has many effects (too many to list here), although
inflammation is an important one. Here are more details on
roles of the CB2 receptor. Like with CB1, THC is a potent partial agonist at the CB2 receptor. CBD opposes activation by THC, since it is an antagonist/inverse agonist. However, it is not particularly potent.
Besides direct actions on the cannabinoid receptors, phytocannabinoids can modulate levels of endocannabinoids. In fact, treatment with CBD increased blood anandamide levels. This could be due to either inhibition of the anandamide-metabolizing enzyme or via inhibition of anandamide reuptake/transport.
Fatty acid amide hydrolase (
FAAH) is the enzyme that metabolizes anandamide. Although inhibition of FAAH by CBD is frequently cited for the increase in anandamide, it not a very potent inhibitor (IC50=15.2 μM). Another study saw very little inhibition of FAAH, even at very high concentrations, bringing into question whether this is how CBD raises anandamide levels.
F
atty acid binding proteins (
FABPs) are intracellular proteins that facilitate the removal of endocannabinoids by shuttling them from the cell membrane to the intracellular enzymes that break them down. Both THC and CBD bind multiple FABPs with Ki (binding affinity) values in the 1 to 3 μM range. THC and CBD can compete with anandamide for binding to the FABPs, which raises anandamide levels. This is the more likely mechanism of how phytocannabinoids raise endocannabinoid levels.
Atypical Cannabinoid Receptors
GPR18 is a G protein-coupled receptor with effects on
blood pressure and
immune function.
THC is a potent full agonist of GPR18 (EC50=960 nM). However, receptor activation is opposed by CBD, which potently inhibited THC-induced actions mediated through GPR18 (IC50=18 nM).
GPR55 activation lowers
blood pressure, is
anti-inflammatory, and can block some types of
pain. GPR55 regulates energy intake and expenditure, which could impact diseases such as
obesity and
diabetes. It is also expressed in bone cells with a possible role in
osteoporosis. GPR55 activation decreased neurodegeneration in models of
multiple sclerosis.
GPR55 is activated by THC under at least some experimental conditions. The potency of THC at GPR55 (EC50=8 nM) was nearly as low as for the CB1 receptor. CBD opposes the activation of GPR55 by acting as a fairly potent antagonist (IC50=445 nM).
So far, I have not seen any studies of THC/CBD and the third atypical cannabinoid receptor,
GPR119.
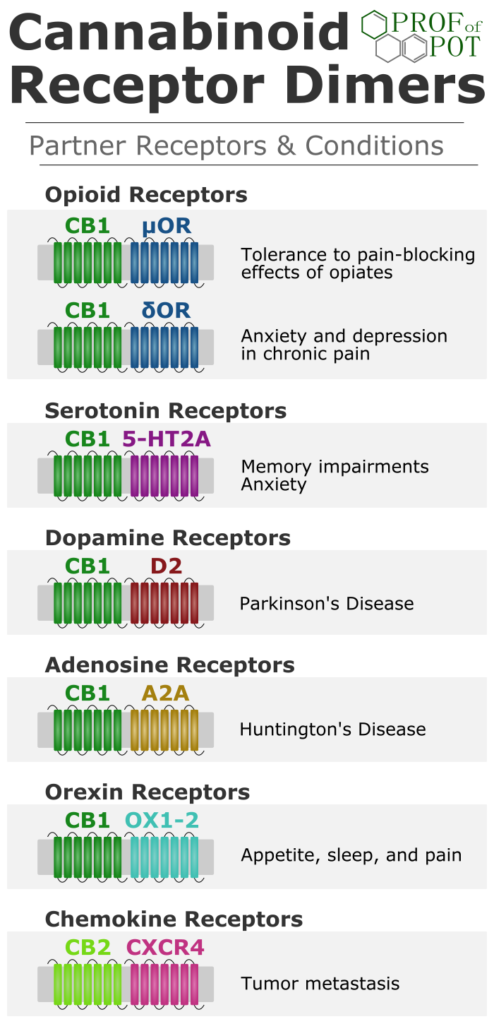
Serotonin Receptors
The
serotonin 5-HT1A receptor is one of the most widespread in the CNS, found in high levels in the cerebral cortex, hippocampus, septum, amygdala, and raphe nucleus. CBD is a full agonist at the 5-HT1A receptor, although with relatively low potency in the microM range. THC, even at high concentrations, did not bind the 5-HT1A receptor. However, both CBD and THC may activate 5-HT1A receptors through indirect mechanisms.
5-HT1A receptor activation is associated with decreased blood pressure, decreased heart rate, and decreased body temperature. 5-HT1A agonist drugs (such as buspirone) can relieve
anxiety and
depression. The 5-HT1A receptor is also important in mediating the anti-depressant effects of SSRIs and even MDMA. 5-HT1A activation is also anti-emetic and analgesic. Finally, 5-HT1A activation may improve symptoms of
schizophrenia and
Parkinson’s Disease. On the other hand, 5-HT1A receptor activation can also cause impaired learning and memory.
The
serotonin 5-HT2A receptor is important for
emotions,
learning, and
memory. THC does not bind the 5-HT2A receptor. CBD is a weak partial agonist, with over 8 μM of CBD required to elicit a significant effect. Since
THC can activate the 5-HT2A receptor through CB1 receptor dimerization, this may be more important than any direct effect of CBD.
The
serotonin 5-HT3 receptor is involved in pain transmission and mood disorders. 5-HT3 antagonists are used for chemotherapy-induced
nausea and
vomiting. Both THC (IC50=38 nM) and CBD (IC50=600 nM) are potent negative allosteric modulator of 5-HT3A. This inhibition may also be partly responsible for the
analgesic and anti-nausea effects of cannabinoids. This
inhibition of the 5-HT3 receptor is shared with endocannabinoid anandamide.
Dopamine Receptors
The
dopamine D2 receptor has a role in many brain functions, but is particularly important in
schizophrenia. Antipsychotic medications act upon the D2 receptor. The D2 receptor can exist in a state of high affinity for dopamine (D2High) or a state of low affinity for dopamine (D2Low). Elevated levels of the D2High receptor are associated with schizophrenia.
CBD has shown antipsychotic properties both by itself and when added to an ongoing treatment regimen. To determine the mechanism through which CBD exerts its antipsychotic effects, the binding of CBD to D2 receptors was tested. CBD was a potent (IC50=66 nM) partial agonist of the D2High receptor, which is a characteristic shared with some other antipsychotics such as aripiprazole.
The partial agonist activity may explain some of the reported side effects of CBD, such as
drowsiness,
diarrhea,
decreased appetite, and
fatigue.
Although binding of THC was not tested, there is no rationale to think that THC would directly interact with D2 receptors. However,
THC can indirectly modulate D2 receptors via receptor dimerization.
Opioid Receptors
Both THC and CBD were reported to be negative allosteric modulators of the
mu opioid receptorand
delta opioid receptor. They decreased binding of opioid ligands, but the potency was quite low (EC50=4-5 μM). However, since only ligand binding and no signaling studies were performed, the significance of this remains unknown.
Dimerization with the CB1 receptor may play a more important role in modulating these opioid receptors than direct modulation by phytocannabinoids.
Adenosine System
Adenosine acts as a signaling molecule both within the brain and outside it. Caffeine, famous for its stimulant effect, is an antagonist of the four
adenosine receptors (
A1,
A2A, A2B, and
A3).
Both THC and CBD can enhance adenosine activity, but they don’t do this by directly interacting with the receptors. After adenosine is released, it is cleared by being transported back inside the cell. Both THC and CBD can potently inhibit adenosine cellular uptake (IC50=270 & 120 nM), leaving more adenosine to activate the receptors. The cannabinoids accomplish this by inhibiting the
equilibrative nucleoside transporter 1 (
ENT1).
Indirect activation of the A1A receptor may mediate the
anti-inflammatory and
immunosuppressive effects of CBD. However, activation of adenosine receptors may also cause
drowsiness and
memory impairment.
Glycine Receptors
Glycine receptors (
GlyRs) are ligand-gated ion channels which inhibit nerve activation. GlyRs regulate both
pain and
inflammation.
Just as
anandamide is a postive allosteric modulator at GlyRs, both THC and CBD can increase channel activation by glycine. The GlyR channel modulation was moderately potent with EC50 values of 2.4 μM (THC) and 2.7 μM (CBD).
Animal studies have shown the importance of cannabinoids acting at these channels to inhibit transmission of pain signals up the spinal cord.
GABA Receptors
The effects of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) are mediated by two receptors, GABA-A and GABA-B.
GABA-A is a ligand-gated ion channel which mediates the effects of barbiturates, benzodiazepines, and alcohol. GABA-A receptors are formed with 5 subunits – each subunit is from one of 19 different genes.
In response to the
anti-seizure activity observed with CBD, interactions with GABA-A were explored. CBD was found to be a positive allosteric modulator of GABA-A. The potency for enhancing GABA effects depended on the subunit configuration of the receptor and ranged from 0.9 to 16.1 μM.
The CBD binding site was separate from the benzodiazepine binding site. CBD showed greater potency at the ß3 subunit, which can produce less
sedation while maintaining the
anxiolyticeffect.
GABA-A modulation by THC was not tested, but there is no strong rationale to think that THC would directly interact like CBD does. Past studies have shown that THC can indirectly activate GABA receptors through inhibition of GABA reuptake. This was dependent on activation of CB1 receptors.
Acetylcholine Receptors
The
nicotinic acetylcholine (
nACh)
receptor is another ligand-gated ion channel consisting of 5 subunits. The α7-nACh receptor (consisting of 5 of the α7 subunits) is expressed in the cerebral cortex, thalamus, and hippocampus and is found on both excitatory and inhibitory nerves. This receptor is involved in
memory,
learning, and
attention and mediates many of the cognitive effects of nicotine. It also has a role in
cancer cell proliferation and
metastasis.
CBD acts as a negative allosteric modulator of the α7-nACh receptor, although it is has low potentency (IC50=11.3 μM). THC did not have any effect on α7-nACh receptors.
TRP Channels
The
transient receptor potential (TRP) channels are ion channels involved in temperature, pressure, and pain sensation. There are three different subfamilies of this class that cannabinoids can modulate: vanilloid (
TRPV), ankyrin (
TRPA), and melastatin (
TRPM).
TRPV1, expressed in sensory neurons and the brain, mediates pain and temperature sensation. It is famous for being activated by capsaicin (the molecule that makes chilis taste spicy). CBD (EC50=1 μM) is an agonist of TRPV1.
But wait, isn’t it bad to activate a receptor that causes a burning pain? Actually, TRPV1 agonists can also cause a prolonged period of receptor desensitization. For this reason, topical capsaicin is used to treat neuropathic pain. At least one model of inflammatory pain showed that the
pain-blocking effects of CBD were due to actions at TRPV1.
The list of related TRP channels acted on by phytocannabinoids is long. THC and CBD both activate
TRPV2 (EC50s=650 nm/ 1.25 μM),
TRPV3 (EC50s=9.5 μM/ 3.7 μM), and
TRPV4(EC50s=8.5 μM/ 800 nM). The potency at
TRPA1 is especially low (EC50s= 230 nM/ 110 nM). Finally, THC and CBD were both potent antagonists of the
TRPM8 channel (EC50s=160 nM/ 60 nM).
We still do not fully understand the role of each of these channels for the effects of phytocannabinoids. I would keep a close eye on research in this area for the treatment of different types of
chronic pain.
Nuclear Receptors
Peroxisome proliferator-activated receptors (
PPARs) are nuclear receptors that regulate gene expression. Activation of PPARs is associated with some of the
neuroprotective,
pain-blocking,
anti-cancer,
anti-inflammatory, and
metabolism-improving properties of cannabinoids.
Although endocannabinoids can activate the
PPARα isoform, evidence for direct activation of PPARα by phytocannabinoids is weak. Activation by THC or CBD may occur indirectly through other receptors.
On the other hand, both THC and CBD are potent and direct activators of the
PPARγ isoform. Although an EC50 has not been calculated, PPARγ activation was seen with just 100 nM of THC or CBD.